Octreotide: Advancements and Applications in Treating Hormone-Related Disorders and Neuroendocrine Tumors
Abstract
Octreotide is a synthetic somatostatin analog widely used in the medical field for its potent inhibitory effects on the release of various hormones. It plays a crucial role in the treatment of acromegaly, a condition characterized by excessive growth hormone production, and gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Octreotide’s mechanism of action involves binding to somatostatin receptors, particularly SST2, resulting in decreased hormone secretion and tumor growth inhibition. Recent advancements include long-acting formulations, such as Octreotide-LAR, which offer improved patient compliance and therapeutic outcomes. Radiolabeled versions of Octreotide are also utilized for diagnostic imaging and targeted radiotherapy, enhancing the precision of cancer treatments. In addition to Octreotide, related compounds such as impurities, metabolites, and isotope-labeled analogs are essential for comprehensive research and development. These compounds aid in understanding Octreotide’s pharmacokinetics, improving therapeutic applications, and developing new treatments. Custom synthesis services further support the creation of specific compounds tailored to research needs. Octreotide continues to be a vital tool in endocrinology and oncology, demonstrating significant benefits for patients with hormone-related disorders and neuroendocrine tumors.
Introduction to Octreotide
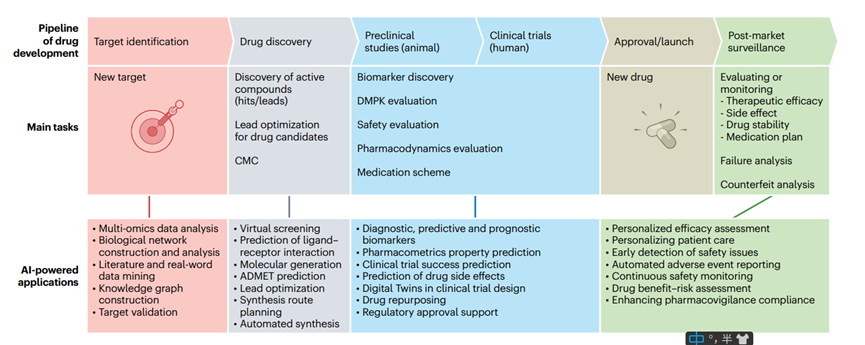
Octreotide is a synthetic analog of somatostatin, a naturally occurring peptide hormone that inhibits the secretion of several other hormones, including growth hormone, insulin, and glucagon. Developed in the early 1980s, Octreotide has since become a cornerstone in the treatment of various endocrine and neuroendocrine disorders. It is particularly significant in managing acromegaly, a condition caused by excessive growth hormone production, and gastroenteropancreatic neuroendocrine tumors (GEP-NETs), which are characterized by hormone-secreting tumors in the gastrointestinal tract and pancreas.
The primary mechanism of action of Octreotide involves binding to somatostatin receptors (SSTRs), especially subtype 2 (SST2), on the surface of target cells. This binding inhibits adenylate cyclase activity, reducing the intracellular levels of cyclic AMP and subsequently decreasing hormone secretion. This targeted approach not only helps in controlling hormone hypersecretion but also exhibits anti-proliferative effects on tumor cells, making Octreotide a valuable therapeutic agent in oncology.
Recent advancements in Octreotide formulations have significantly improved its clinical utility. Long-acting formulations, such as Octreotide LAR (long-acting repeatable), have been developed to provide sustained drug release, reducing the frequency of administration and enhancing patient compliance. Additionally, radiolabeled forms of Octreotide, such as 68Ga-DOTATATE, are increasingly used in diagnostic imaging and targeted radiotherapy for neuroendocrine tumors. These innovations enable precise localization of tumors and targeted delivery of therapeutic radiation, improving treatment outcomes.
Octreotide is also crucial in the management of various other conditions, including severe diarrhea associated with certain tumors, bleeding esophageal varices, and hyperinsulinism. Its versatility and effectiveness across multiple indications underscore its importance in both endocrinology and oncology.
Despite its widespread use, ongoing research continues to explore new applications and optimize existing therapies involving Octreotide. Studies are investigating its potential benefits in other hypersecretory conditions and further improving its pharmacokinetic properties to enhance patient outcomes.
Mechanism of Action and Uses
Octreotide, a potent synthetic analog of somatostatin, exerts its therapeutic effects through the inhibition of hormone secretion. Somatostatin, the naturally occurring hormone, regulates the endocrine system and inhibits the release of growth hormone (GH), insulin, glucagon, and other peptides. Octreotide mimics these inhibitory effects by binding to somatostatin receptors (SSTRs) on the surface of target cells, particularly SSTR2. This binding reduces intracellular cyclic AMP levels by inhibiting adenylate cyclase activity, thereby decreasing hormone secretion.

One of the primary uses of Octreotide is in the treatment of acromegaly, a disorder characterized by excessive GH production, usually due to a benign pituitary tumor. By binding to SSTR2 on pituitary adenomas, Octreotide effectively reduces GH secretion, leading to normalization of insulin-like growth factor 1 (IGF-1) levels and alleviation of symptoms associated with acromegaly, such as enlarged hands and feet, facial changes, and joint pain. Long-term studies have demonstrated that Octreotide significantly improves quality of life and reduces comorbidities in acromegaly patients.
Octreotide is also widely used in managing gastroenteropancreatic neuroendocrine tumors (GEP-NETs), which secrete various peptides and hormones causing symptoms like diarrhea, flushing, and wheezing. Octreotide helps control these symptoms by inhibiting hormone release from the tumors. Its long-acting formulation, Octreotide LAR, has made chronic management of GEP-NETs more convenient and effective by reducing the frequency of administration to once a month.
The efficacy of Octreotide extends beyond hormonal control. It exhibits anti-proliferative effects on tumor cells, contributing to tumor growth inhibition. This effect is particularly beneficial in GEP-NET patients, where Octreotide not only alleviates symptoms but also slows tumor progression. Additionally, radiolabeled forms of Octreotide, such as 68Ga-DOTATATE, are used in diagnostic imaging to locate neuroendocrine tumors and assess their receptor status, enabling precise targeted therapy.
In terms of safety, Octreotide is generally well-tolerated. Common side effects include gastrointestinal disturbances such as nausea, abdominal pain, and diarrhea, which typically resolve with continued use. Long-term safety profiles indicate that Octreotide does not cause significant adverse effects, making it a reliable option for prolonged treatment in endocrine disorders.
Advances and Innovations
Recent advancements in Octreotide formulations have significantly enhanced its clinical utility and patient outcomes. One of the most notable innovations is the development of long-acting formulations such as Octreotide LAR (long-acting repeatable). This formulation allows for a sustained release of the drug, requiring administration only once a month compared to the daily injections of the immediate-release form. The extended release not only improves patient compliance but also provides consistent therapeutic effects, maintaining stable hormone levels and better disease control.
Another significant advancement is the use of radiolabeled Octreotide in both diagnostic imaging and targeted radiotherapy. Compounds like 68Ga-DOTATATE and 177Lu-DOTATATE have revolutionized the management of neuroendocrine tumors (NETs). 68Ga-DOTATATE is used in positron emission tomography (PET) imaging to accurately localize NETs and assess their receptor status. This imaging technique provides high sensitivity and specificity, allowing for precise tumor detection and staging.
In the realm of targeted radiotherapy, 177Lu-DOTATATE has shown remarkable efficacy in treating advanced, inoperable NETs. This radiopharmaceutical binds to somatostatin receptors on the tumor cells, delivering targeted radiation that induces tumor cell death while sparing surrounding healthy tissue. Clinical trials have demonstrated that 177Lu-DOTATATE significantly improves progression-free survival and overall survival in patients with metastatic NETs, offering a promising treatment option for a challenging condition.
Beyond these clinical applications, research continues to explore the potential of Octreotide in new therapeutic areas. For instance, studies are investigating its use in managing severe diarrhea associated with certain tumors and conditions, bleeding esophageal varices, and congenital hyperinsulinism. These studies aim to expand the therapeutic indications of Octreotide, providing more options for patients with various endocrine and gastrointestinal disorders.
Furthermore, the development of novel Octreotide analogs with enhanced receptor binding affinities and improved pharmacokinetic profiles is underway. These next-generation analogs aim to provide even greater efficacy, reduced side effects, and more convenient dosing schedules, further improving patient outcomes and quality of life.
The continuous innovation in Octreotide formulations and applications underscores its importance in modern medicine. These advancements not only enhance the therapeutic potential of Octreotide but also pave the way for new treatment paradigms in endocrine and oncologic care, benefiting a wide range of patients.
Related Compounds and Custom Synthesis
In addition to Octreotide, a range of related compounds play crucial roles in pharmaceutical research and development. These include impurities, metabolites, isotope-labeled compounds, and building blocks, each essential for various stages of drug discovery and development. Impurities and metabolites are vital for understanding the safety and efficacy of Octreotide by identifying potential side effects and metabolic pathways. Isotope-labeled compounds, such as deuterium-labeled Octreotide, enable detailed pharmacokinetic and pharmacodynamic studies, providing insights into the drug’s behavior in the body.
Building blocks, on the other hand, are fundamental in the synthesis of more complex molecules, facilitating the development of new therapeutic analogs and derivatives. These compounds help in enhancing the drug’s properties, such as its stability, efficacy, and safety profile.
Custom synthesis services are available to support researchers in obtaining specific compounds tailored to their unique research needs. These services offer high-purity, precisely characterized chemicals that integrate seamlessly into existing research protocols. Technical support from experts ensures that the synthesized compounds meet the stringent requirements of pharmaceutical research, thereby advancing the development of novel therapies and improving patient outcomes.
Conclusion
Octreotide remains a pivotal therapeutic agent in the management of hormone-related disorders and neuroendocrine tumors. Its ability to inhibit hormone secretion and exert anti-proliferative effects on tumor cells has made it indispensable in treating conditions such as acromegaly and gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Innovations like the long-acting Octreotide LAR and radiolabeled versions for diagnostic imaging and targeted radiotherapy have significantly enhanced its clinical applications, offering better patient compliance and improved treatment outcomes.
The availability of related compounds, including impurities, metabolites, isotope-labeled compounds, and building blocks, further supports advanced research and development efforts. These compounds are crucial for understanding the pharmacokinetics and pharmacodynamics of Octreotide, optimizing its therapeutic use, and developing new treatment options. Custom synthesis services and technical support play a vital role in providing researchers with high-quality, precisely characterized chemicals, ensuring the success of their scientific investigations.
As ongoing research continues to explore new applications and optimize existing therapies, Octreotide’s role in modern medicine is expected to expand even further. Its versatility and efficacy underscore its importance in endocrinology and oncology, offering hope and improved quality of life for patients with complex medical conditions.
Reference
- Lamberts, S. W., & Hofland, L. J. (2019). ANNIVERSARY REVIEW: Octreotide, 40 years later. European journal of endocrinology, 181(5), R173-R183.
- Hofland, L. J., & Lamberts, S. W. (2003). The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocrine reviews, 24(1), 28-47.
- Colao, A., Grasso, L. F., Giustina, A., Melmed, S., Chanson, P., Pereira, A. M., & Pivonello, R. (2019). Acromegaly. Nature Reviews Disease Primers, 5(1), 20.
- Stajich, G., & Ashworth, L. (2006). Octreotide. Neonatal Network, 25(5), 365-369.