Ojemda (Tovorafenib): A Game-Changer in Targeted Therapy for Pediatric Low-Grade Gliomas
Abstract
Ojemda (tovorafenib) is an FDA-approved treatment for pediatric low-grade gliomas (pLGG) harboring BRAF mutations. Its unique mechanism of action as a type II RAF kinase inhibitor allows it to target both BRAF fusions and V600 mutations, offering a broader treatment application than other therapies. Clinical trials, particularly the FIREFLY-1 study, have demonstrated a significant overall response rate (ORR) of 51%, with durable tumor control lasting for a median of 13.8 months. Ongoing research, such as the FIREFLY-2 trial, aims to expand its use as a front-line therapy. Additionally, combination studies with MEK inhibitors may further extend its applicability to a broader range of cancers involving the MAPK pathway. Ojemda’s once-weekly dosing and manageable side-effect profile make it an appealing option in pediatric oncology.
Ojemda (Tovorafenib): Breakthrough in Pediatric Glioma Treatment
Ojemda, also known as tovorafenib, is a novel treatment recently approved by the U.S. FDA for pediatric low-grade gliomas (pLGG) that harbor specific BRAF mutations. As the most common type of brain tumor in children, pLGG has historically had limited systemic treatment options, particularly for cases that relapse or resist initial therapies. Ojemda represents a significant advancement in addressing these cases, targeting BRAF alterations such as BRAF fusions and the V600 mutation, which are commonly implicated in tumor growth.
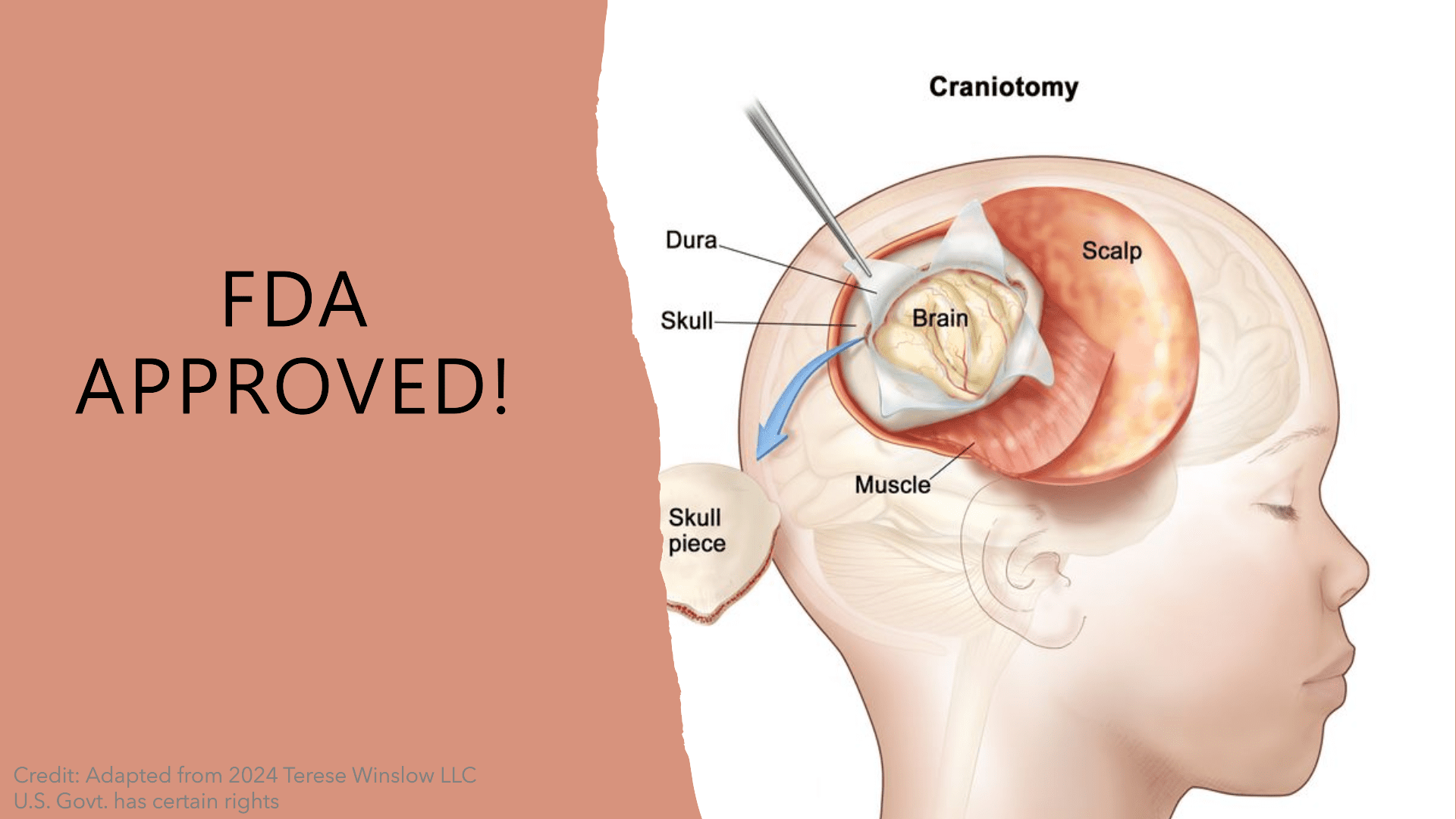
Fig.1 FDA Approval of Ojemda
Tovorafenib works as a type II RAF kinase inhibitor, blocking abnormal BRAF proteins that fuel the progression of gliomas. This makes it an essential treatment option for children aged 6 months and older who have not responded to surgery or previous therapies. Unlike earlier treatments that focus on specific BRAF mutations, Ojemda effectively targets both BRAF fusion proteins and V600 mutations, offering a broader application compared to other therapies such as the Tafinlar-Mekinist combination.
The approval of Ojemda was supported by the Phase II FIREFLY-1 trial, which demonstrated a 51% overall response rate in patients with relapsed or refractory pLGG. This impressive efficacy, combined with its manageable side-effect profile, positions Ojemda as a promising new option for pediatric patients. Its once-weekly oral dosing further enhances its appeal by reducing the treatment burden for young patients and their families.
Ongoing studies, such as FIREFLY-2, aim to further investigate Ojemda as a potential first-line therapy, which could expand its role in treating childhood brain cancers. With this breakthrough, Ojemda marks a new era in pediatric oncology, offering hope to children and families facing this challenging diagnosis.
Mechanism of Action: Targeting BRAF Mutations
Ojemda (tovorafenib) is a type II RAF kinase inhibitor designed to target specific mutations in the BRAF gene, which are often involved in the development and progression of pediatric low-grade gliomas (pLGG). This drug specifically inhibits mutant forms of BRAF, including BRAF V600 and BRAF fusion proteins, both of which are common in pediatric gliomas. Unlike type I BRAF inhibitors, which can inadvertently cause tumor growth in cases of BRAF fusions, tovorafenib’s mechanism of action allows it to block the harmful signaling pathways without causing this paradoxical activation.
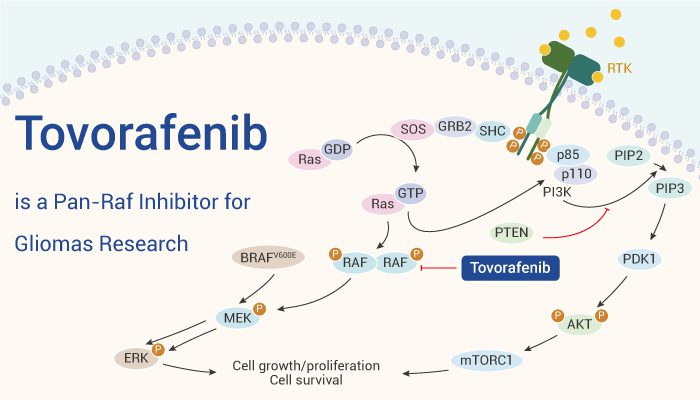
Fig.2 Tovorafenib:Mechanism of Action
BRAF mutations play a crucial role in activating the MAPK signaling pathway, which controls cell growth and survival. In gliomas, mutations in BRAF can cause uncontrolled cell division, leading to tumor formation. Ojemda’s action effectively inhibits this pathway, thereby reducing tumor proliferation and inducing tumor shrinkage. By targeting both BRAF fusions and V600 mutations, Ojemda provides a broader therapeutic approach than existing treatments like Tafinlar (dabrafenib) and Mekinist (trametinib), which are effective only for the V600 mutation.
Ojemda is taken once weekly, offering a convenient dosing schedule that minimizes treatment disruptions for pediatric patients. This simplicity, combined with its ability to inhibit a wide range of BRAF mutations, makes it a highly versatile option for managing pediatric gliomas. Ongoing studies continue to explore the efficacy of combining Ojemda with other targeted therapies like MEK inhibitors, which may further enhance its therapeutic benefits.
Clinical Trials: Efficacy and Safety of Ojemda (Tovorafenib)
Ojemda (tovorafenib) earned its FDA approval based on promising results from the Phase II FIREFLY-1 clinical trial, which evaluated the drug’s efficacy and safety in pediatric patients with low-grade gliomas (pLGG) harboring BRAF mutations. The trial focused on patients who had relapsed or were refractory to previous treatments, demonstrating the urgent need for effective therapeutic options. The FIREFLY-1 trial enrolled 76 patients, all of whom had a documented BRAF fusion or V600 mutation, a key target for tovorafenib’s mechanism of action.
The primary outcome of the trial was the overall response rate (ORR), defined as the proportion of patients who achieved a complete, partial, or minor response to treatment. Ojemda showed an ORR of 51%, with tumor shrinkage observed in more than half of the patients. The response was particularly strong in those with BRAF fusions, with a response rate of 52%, and in patients with the BRAF V600 mutation, with a response rate of 50%. These results are especially encouraging given that low-grade gliomas often have a more indolent course, and achieving significant tumor shrinkage in relapsed patients is challenging.
Another crucial outcome measure was the duration of response (DoR), which was 13.8 months at the median. This indicates that tovorafenib’s therapeutic effect was not only robust but also long-lasting, providing patients with an extended period of tumor control before any potential progression. Moreover, the safety profile of tovorafenib was favorable, with most side effects being manageable and non-life-threatening. The most common side effects reported were changes in hair color, fatigue, viral infections, rash, and gastrointestinal symptoms such as vomiting and constipation.
It is important to note that the side effects of Ojemda are consistent with those expected from a targeted therapy that inhibits the RAF kinases. For example, hair color changes, a commonly reported side effect, result from the inhibition of wild-type BRAF, which plays a role in pigmentation. Similarly, the gastrointestinal side effects observed are typical for therapies that target the MAPK pathway. In severe cases, however, patients experienced anemia, increased creatinine phosphokinase, and elevated liver enzymes, which led to early discontinuation of the treatment in 9 participants. This underlines the need for continuous monitoring and supportive care in managing treatment-related toxicities.
The efficacy of Ojemda, as demonstrated by its ability to shrink tumors and prolong response duration, along with its relatively manageable side effect profile, positions it as a valuable treatment option for pediatric gliomas. It offers a new hope for patients with relapsed or refractory gliomas who have exhausted other therapeutic options. Additionally, its once-weekly dosing regimen improves the quality of life for pediatric patients, reducing the frequency of hospital visits and the burden of treatment.
Comparing Ojemda with Other Treatments
In the field of pediatric glioma treatment, Ojemda stands out not only for its efficacy but also for its unique ability to target both BRAF fusions and V600 mutations. This broad action distinguishes it from other available treatments, such as the Tafinlar-Mekinist combination therapy, which is approved for use in BRAF V600 mutations but does not effectively target BRAF fusions. This is a key distinction because BRAF fusions are more prevalent in pediatric low-grade gliomas, accounting for about 80% of BRAF alterations in this population.
The Tafinlar-Mekinist combination works by inhibiting BRAF and MEK kinases, components of the same MAPK signaling pathway that is dysregulated in many gliomas. However, because Tafinlar (dabrafenib) is a type I BRAF inhibitor, it specifically blocks the V600 form of the protein, which can cause paradoxical activation of the MAPK pathway in tumors with BRAF fusions. This phenomenon can lead to enhanced tumor growth, making Tafinlar less effective for patients with BRAF fusions. By contrast, Ojemda’s type II inhibition mechanism allows it to block both BRAF V600 and fusion proteins, without triggering this paradoxical effect, thus offering a broader and safer application.
Another significant advantage of Ojemda is its simplified dosing schedule. Patients take Ojemda once weekly, in contrast to the twice-daily regimen required for Tafinlar. This not only reduces the treatment burden on young patients but also minimizes the potential for missed doses, making it easier to manage in a pediatric setting. Additionally, Ojemda’s approval does not include a boxed warning, unlike many other cancer therapies, further reinforcing its relatively favorable safety profile.
Ojemda is also being studied in combination with other therapies, such as the MEK inhibitor pimasertib, in clinical trials for adolescent and adult patients with recurrent or progressive solid tumors harboring MAPK pathway alterations. These studies may expand Ojemda’s therapeutic applications beyond pediatric gliomas, potentially making it a versatile treatment option for a range of cancers driven by MAPK pathway dysregulation.
Finally, the ongoing FIREFLY-2 trial is comparing Ojemda as a first-line treatment for pediatric gliomas against standard chemotherapy. Early results from this study could pave the way for Ojemda’s expanded use as an initial treatment, not just for relapsed or refractory cases. This would further cement its position as a cornerstone therapy for pediatric gliomas and possibly influence treatment guidelines in the future.
Future Directions: Ongoing Research and Expanding Use of Ojemda (Tovorafenib)
Ojemda (tovorafenib) is being actively studied in ongoing research to assess its potential beyond its current indication for relapsed or refractory pediatric low-grade glioma (pLGG). One of the most promising trials is FIREFLY-2, a Phase 3 study aimed at evaluating Ojemda as a front-line therapy compared to standard chemotherapy in pediatric patients with newly diagnosed pLGG. The results of this trial could significantly expand the drug’s use, potentially making it a first-line treatment option for children who have not yet undergone other therapies.
Moreover, Ojemda is also being explored in combination with other targeted therapies. For example, researchers are evaluating the combination of Ojemda with the MEK inhibitor pimasertib for adolescent and adult patients with recurrent or progressive solid tumors that exhibit MAPK pathway alterations. This combination could extend the utility of Ojemda to other cancers, particularly those driven by BRAF mutations and related genetic alterations.
The versatility of Ojemda lies in its unique mechanism of action, which allows it to target a broad spectrum of BRAF alterations, including both V600 mutations and fusions. This makes it a valuable asset in the ongoing fight against cancers that rely on MAPK pathway dysregulation for survival and proliferation. As research continues, the potential applications of Ojemda could broaden, offering new hope for patients across various age groups and cancer types.
References
- Dhillon S. Tovorafenib: First Approval. Drugs. 2024 Aug;84(8):985-993. doi: 10.1007/s40265-024-02069-6. Epub 2024 Jul 5. PMID: 38967715.
- Khoury JE, Wehbe S, Attieh F, Boutros M, Kesrouani C, Kourie HR. A critical review of RAF inhibitors in BRAF-mutated glioma treatment. Pharmacogenomics. 2024;25(7):343-355. doi: 10.1080/14622416.2024.2355859. Epub 2024 Jun 3. PMID: 38884947; PMCID: PMC11404696.