Optimizing Pharmaceutical Research: Easy-to-Implement Hydrogen Isotope Exchange
Abstract
Hydrogen Isotope Exchange (HIE) is an essential technique in pharmaceutical research, enabling the synthesis of deuterated and tritiated compounds. These isotopically labeled molecules play a crucial role in drug development and scientific research, particularly in mass spectrometry and ADME studies. Traditional HIE methods face challenges such as harsh reaction conditions, poor selectivity, and limited functional group tolerance. Recent advancements, notably the development of rhodium (Rh) nanoparticles, have significantly improved the efficiency and applicability of HIE. Rh nanoparticles enable selective labeling of complex molecules under mild conditions, achieving high isotopic enrichment while preserving the integrity of sensitive pharmaceuticals. This innovative approach has been successfully applied to a variety of substrates, including indole, nicotine, and chloroquine, demonstrating its broad applicability. The Rh nanoparticle method offers substantial benefits over traditional techniques, providing a robust, cost-effective, and efficient means of producing labeled pharmaceuticals. Ongoing research aims to further refine this method, exploring its potential for broader applications in drug development, material science, and biochemical studies. As this technique gains traction, it promises to become a standard tool in pharmaceutical research, facilitating the development of new drugs and advanced materials.
Keywords: Hydrogen Isotope Exchange (HIE), Rhodium Nanoparticles, Deuterated Compounds, Tritiated Compounds, Pharmaceutical Labeling
Introduction
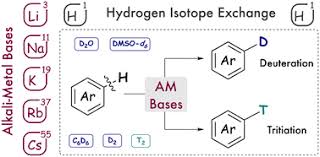
Hydrogen Isotope Exchange (HIE) is a pivotal technique in the field of pharmaceutical research. This process involves the replacement of hydrogen atoms in a molecule with its isotopes, deuterium or tritium. The resulting isotopically labeled compounds are invaluable in various stages of drug development and scientific research.
HIE plays a crucial role in the synthesis of deuterated and tritiated compounds, which are extensively used in drug discovery and development. Deuterium, a stable isotope of hydrogen, is commonly incorporated into drug molecules to create deuterated analogs. These analogs serve as internal standards in mass spectrometry, facilitating the accurate quantification of drugs and their metabolites. Additionally, the incorporation of deuterium can enhance the pharmacokinetic properties of drugs by reducing their metabolic rate, thereby increasing their half-life and improving their safety profiles. This leads to the development of so-called “heavy drugs,” which require lower dosages and exhibit fewer side effects.
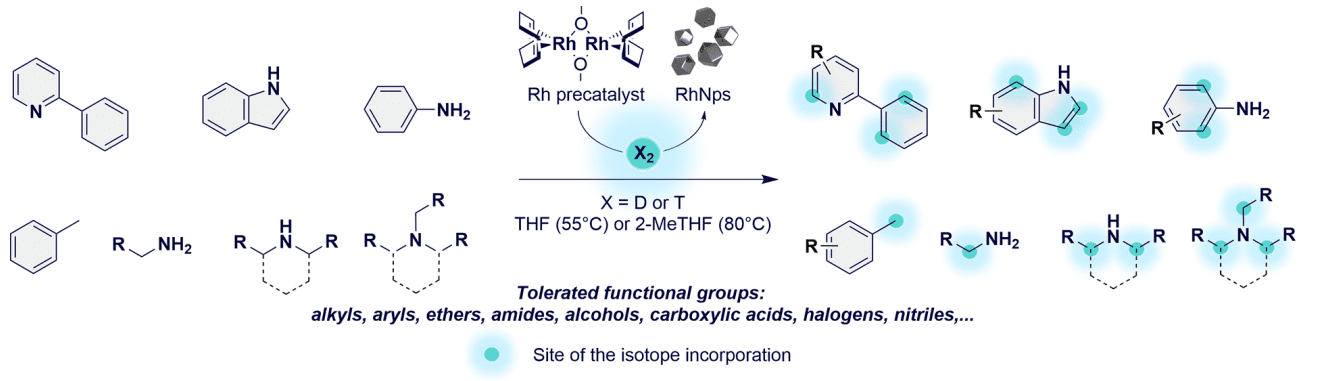
Figure 1 Substrate scope and regioselectivity of the isotope incorporation.
Tritiated compounds, which contain the radioactive isotope tritium, are essential in preclinical studies. They are used in absorption, distribution, metabolism, and excretion (ADME) studies to track the behavior of drug candidates within biological systems. The high sensitivity of tritium allows for the detection and quantification of drugs at very low concentrations, providing detailed insights into their pharmacokinetics and pharmacodynamics.
The advancements in HIE techniques have addressed several challenges associated with traditional methods. Conventional HIE methods often involve harsh reaction conditions, limited selectivity, and poor functional group tolerance. However, recent innovations, such as the use of rhodium (Rh) nanoparticles, have significantly improved the efficiency and applicability of HIE. These nanoparticles enable the selective labeling of complex molecules under mild conditions, making the process more versatile and accessible for pharmaceutical research.
In summary, HIE is an indispensable tool in pharmaceutical research, enabling the creation of deuterated and tritiated compounds that are critical for drug development and ADME studies. The continuous evolution of HIE methodologies promises to further enhance the precision and efficiency of drug discovery processes.
Challenges with Existing HIE Methods
Hydrogen Isotope Exchange (HIE) methods have historically faced several significant challenges, limiting their broader application in pharmaceutical research. Traditional HIE techniques often require harsh reaction conditions, which can damage sensitive functional groups in complex molecules. These conditions, such as high temperatures and strong acids or bases, restrict the range of compounds that can be effectively labeled, thereby limiting the versatility of HIE in drug development.
One of the primary issues with conventional HIE methods is their poor selectivity. These methods frequently result in non-specific labeling, where isotopes are incorporated at multiple sites within the molecule. This lack of precision complicates the interpretation of experimental results and reduces the utility of labeled compounds in specific applications such as pharmacokinetic studies and mass spectrometry.
Functional group tolerance is another critical limitation of traditional HIE techniques. Many pharmaceuticals contain a variety of functional groups that can react differently under the conditions required for HIE. For instance, certain functional groups may undergo side reactions, leading to the formation of undesired by-products. This problem is particularly pronounced in the labeling of complex drug molecules, which often feature multiple reactive sites.
Furthermore, achieving high isotopic enrichment with traditional HIE methods can be challenging. High levels of isotopic incorporation are essential for many applications, such as creating internal standards for mass spectrometry and developing deuterated drugs with enhanced pharmacokinetic properties. However, traditional methods often fall short in this regard, resulting in products with suboptimal isotopic enrichment.
Recent advancements have aimed to address these challenges. Innovations such as the use of rhodium (Rh) nanoparticles have shown promise in overcoming the limitations of traditional HIE methods. Rh nanoparticles enable selective labeling under mild conditions, significantly improving functional group tolerance and isotopic enrichment. These advancements not only broaden the applicability of HIE but also enhance its precision and efficiency, making it a more viable tool for modern pharmaceutical research.
In summary, while traditional HIE methods have played a crucial role in pharmaceutical research, their limitations in terms of reaction conditions, selectivity, functional group tolerance, and isotopic enrichment have prompted the development of more advanced techniques. The advent of nanoparticle-based HIE methods marks a significant step forward, addressing these challenges and opening new possibilities for the synthesis of labeled compounds.
Innovative Rh Nanoparticle Approach
Development and Methodology
Recent advancements in hydrogen isotope exchange (HIE) have been spearheaded by the development of rhodium (Rh) nanoparticles. This innovative approach addresses many of the limitations associated with traditional HIE techniques, offering a more efficient and selective method for the labeling of pharmaceuticals.
Formation and Properties of Rh Nanoparticles
Rh nanoparticles are generated in situ by the decomposition of a commercially available rhodium dimer under a deuterium or tritium gas atmosphere. This process takes place under mild conditions, typically at room temperature and low pressures, making it compatible with a wide range of pharmaceutical substrates. The nanoparticles formed are small, with a mean diameter of around 3 nm, and display remarkable stability and reactivity, which are critical for effective HIE.
Experimental Setup: Conditions, Substrates, and Process Details
The experimental setup for Rh nanoparticle-mediated HIE involves using the substrate itself to control the aggregation state of the nanoparticles, which acts as a surface ligand. This control ensures the formation of well-dispersed and homogeneous nanoparticles, enhancing their catalytic efficiency. Various substrates, including N-heterocycles, alkylamines, and benzylic scaffolds, have been successfully labeled using this approach, demonstrating its broad applicability.
Control of Nanoparticle Aggregation by Substrates
One of the key advantages of this method is the ability to control nanoparticle aggregation by using the substrates as stabilizing agents. This prevents the formation of large aggregates and maintains the nanoparticles’ high surface area, which is essential for effective catalysis. For instance, alkylamines and N-heterocycles can stabilize the Rh nanoparticles, ensuring a consistent size and dispersion, which leads to efficient and selective HIE.
The innovative Rh nanoparticle method provides a robust solution to the challenges of traditional HIE techniques. By operating under mild conditions and achieving high isotopic enrichment with excellent functional group tolerance, this method facilitates the labeling of complex pharmaceutical compounds, paving the way for its widespread application in drug development and material science.
Applications in Pharmaceutical Labeling
Successful Deuteration and Tritiated Labeling
The innovative use of Rh nanoparticles for hydrogen isotope exchange (HIE) has demonstrated significant potential in the labeling of pharmaceuticals. This method has proven effective for deuteration and tritiation of various drug molecules, providing essential tools for drug development and research.
Examples of Model Substrates and Complex Pharmaceuticals
Several model substrates and complex pharmaceuticals have been successfully labeled using the Rh nanoparticle approach. Notable examples include indole, nicotine, and chloroquine. Indole, a common structural motif in many biologically active compounds, was efficiently deuterated at specific positions, showcasing the method’s precision. Nicotine, a compound of interest in both medical and toxicological studies, was labeled with high isotopic enrichment, enhancing its utility in pharmacokinetic studies. Chloroquine, an antimalarial drug, also demonstrated successful incorporation of deuterium and tritium, underscoring the method’s applicability to diverse pharmaceutical compounds.
Efficiency, Isotopic Enrichment, and Benefits of the Method
The efficiency of the Rh nanoparticle method is highlighted by its ability to achieve high isotopic enrichment under mild reaction conditions. This approach not only facilitates the incorporation of isotopes at desired positions but also preserves the structural integrity of sensitive pharmaceuticals. Compared to traditional HIE techniques, which often require harsh conditions and result in poor selectivity, the Rh nanoparticle method operates under low temperatures and pressures, making it suitable for a wider range of compounds. Furthermore, the method’s high functional group tolerance allows for the labeling of complex molecules without the need for extensive protective group strategies.
The benefits of this method are substantial. It provides a straightforward, cost-effective, and efficient means of producing deuterated and tritiated pharmaceuticals, which are crucial for various analytical and therapeutic applications. The high isotopic enrichment achieved with this method ensures the reliability of labeled compounds in scientific studies, thereby advancing pharmaceutical research and development.
Conclusion and Future Directions
The innovative Rh nanoparticle approach for hydrogen isotope exchange (HIE) offers significant advantages over traditional methods, positioning itself as a transformative tool in pharmaceutical research. This method provides a robust, efficient, and selective means of labeling complex pharmaceuticals, addressing many of the limitations that have historically hampered HIE techniques.
Compared to traditional HIE methods, the Rh nanoparticle approach operates under milder reaction conditions, such as lower temperatures and pressures, making it suitable for a wider range of compounds. The method’s high selectivity and functional group tolerance ensure that isotopic labeling occurs precisely at the desired positions without damaging sensitive pharmaceutical molecules. Additionally, the Rh nanoparticle method achieves higher isotopic enrichment, resulting in more reliable labeled compounds for scientific studies. This efficiency and selectivity make it a superior alternative to traditional HIE techniques, which often require harsh conditions and offer poor selectivity and isotopic enrichment.
Beyond pharmaceutical labeling, the Rh nanoparticle method has the potential to impact other scientific fields. In material science, for instance, deuterated compounds are used to improve the stability and performance of materials such as organic light-emitting diodes (OLEDs) and polymers. The method’s ability to efficiently label a variety of substrates suggests that it could facilitate the development of advanced materials with enhanced properties. Furthermore, the method’s precision and efficiency could be leveraged in the synthesis of isotopically labeled compounds for use in biochemical and environmental studies.
Ongoing research is focused on further refining the Rh nanoparticle method to enhance its efficiency and broaden its applicability. Future studies aim to optimize the reaction conditions and explore the method’s potential for labeling even more complex molecules. Additionally, researchers are investigating the recyclability of Rh nanoparticles, which could improve the method’s sustainability and cost-effectiveness. The continued development and refinement of this technique promise to expand its applications, encouraging its adoption in both research and commercial settings. As the method gains traction, it is likely to become a standard tool in pharmaceutical research, facilitating the development of new drugs and materials.