Ozanimod (Zeposia): A Comprehensive Guide
Abstract
An important development in the management of recurrent multiple sclerosis (MS) and moderately to highly active ulcerative colitis (UC) is the oral drug ozanimod, also known as Zeposia. It works by regulating sphingosine 1-phosphate (S1P) receptors in a selective manner, which minimizes systemic adverse effects and leads to focused immune suppression and decreased disease activity. Its effectiveness in reducing MS relapse rates and causing UC remission has been shown in clinical trials, which enhances patient quality of life.
Introduction to Ozanimod
Sold under the brand name Zeposia, ozanimod is a novel oral drug that has revolutionized the way some autoimmune diseases are treated. It has been approved by regulatory bodies like the FDA and is recommended for adults with moderately to severely active ulcerative colitis (UC) and relapsing forms of multiple sclerosis (MS). Its unique mode of action and good safety record have made it a valuable treatment option for patients looking for a convenient and effective treatment.
In autoimmune disorders such as multiple sclerosis (MS) and ulcerative colitis (UC), the body’s immune system mistakenly targets healthy cells, resulting in persistent inflammation and a variety of incapacitating symptoms. Injectable therapies or drugs with wide immunosuppressive effects are frequently used in traditional treatments, although they can have serious adverse effects and be very inconvenient. By selectively modifying the immune response, Ozanimod presents a novel strategy for lowering inflammation with the least amount of systemic side effects.
Uses and Mechanism of Action of Ozanimod
Sphingosine 1-phosphate (S1P) receptor modulator ozanimod has gained prominence for its ability to effectively treat a number of autoimmune diseases, including relapse forms of MS and moderately to highly active ulcerative colitis (UC). Ozanimod provides a more refined approach to immune modulation by focusing on particular S1P receptor subtypes, with the goal of reducing disease activity while decreasing systemic side effects.
Approved Uses
Adults with relapsing types of multiple sclerosis (MS), such as clinically isolated syndrome, relapsing-remitting MS, and active secondary progressive MS, can take Ozanimod. According to clinical trials, ozanimod lowers the annualized relapse rate and the occurrence of new or expanding brain lesions in MS patients, two key markers of the disease’s progression.
Ulcerative Colitis (UC): Ozanimod has been demonstrated to both initiate and sustain clinical remission in patients with moderately to severely active UC. With its approval, patients with UC who might not respond well to traditional medications now have a new oral therapeutic option.
Mechanism of Action
Ozanimod binds specifically to S1P receptor subtypes 1 and 5 in order to produce its therapeutic effects. The surface of lymphocytes, a subset of white blood cells implicated in immunological responses, expresses these receptors. Through its modulation of these receptors, ozanimod causes the receptors to internalize and degrade, so effectively trapping lymphocytes inside lymph nodes and stopping their migration to inflammatory areas in the gastrointestinal tract and central nervous system.
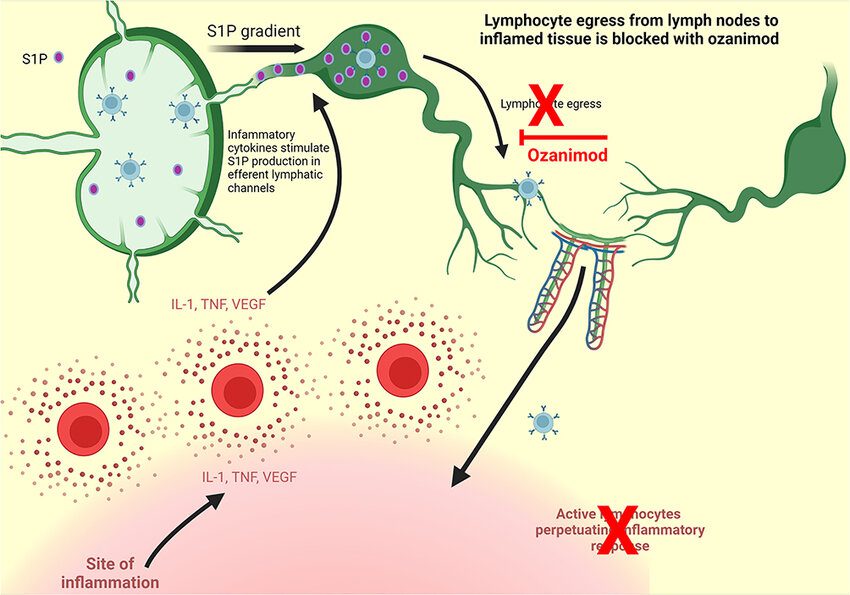
Fig.1 Ozanimod mechanism of Action
Due to the lymphocytes’ sequestration, there are fewer circulating lymphocytes, which lessens the immune system’s erroneous attacks on myelin in MS and the colonic mucosa in UC. Ozanimod has a better safety profile than non-selective S1P modulators because of its receptor selectivity, which is intended to reduce effects on other S1P receptors linked to harmful cardiac and pulmonary consequences.
Benefits
Efficacy in Clinical Trials
Multiple Sclerosis: Compared to interferon beta-1a, the conventional treatment for MS, ozanimod dramatically reduces annualized recurrence rates and the formation of new or expanding brain lesions, according to clinical studies like the SUNBEAM and RADIANCE trials. Patients on ozanimod also exhibited fewer gadolinium-enhancing MRI lesions, indicating lower disease activity.
Ulcerative Colitis: During both the induction and maintenance stages of the True North trial, ozanimod demonstrated greater rates of clinical remission and mucosal healing in comparison to placebo. Patients’ reports of decreased frequency of stools and rectal bleeding led to better illness treatment.
Quality of Life Improvements
When compared to injectable therapy, oral administration of ozanimod enhances patient adherence and convenience. Lower relapse rates and slower disability progression translate into continued mobility and independence for MS patients. Less gastrointestinal discomfort benefits UC patients, improving their nutritional condition and general well-being. Because of the medication’s focused action, the patient’s quality of life is improved by less systemic adverse effects.
Ozanimod is a major breakthrough in the treatment of MS and UC, providing efficient disease control with an easy-to-follow dosage schedule. For people looking for cutting-edge therapy approaches, it is a worthwhile choice due to its advantages in terms of clinical efficacy and patient quality of life.
Side Effects and Precautions of Ozanimod
Marketed as Zeposia, ozanimod is generally well-tolerated; but, as with all drugs, patients and healthcare providers should be informed of potential adverse effects and precautions. Comprehending these facets is vital in order to optimize therapeutic advantages while reducing hazards.
Common Side Effects
- The following are the ozanimod adverse effects that are most commonly reported:
- Headache: Mild to moderate headaches are possible in patients, however they are typically temporary.
- Fatigue: Feelings of exhaustion or sluggishness may arise, which may affect day-to-day activities.
- Upper Respiratory Infections: A lower lymphocyte count that affects the immune response might cause symptoms like a sore throat or nasal congestion.
Dangerous Side Effects
- Even though they are less frequent, more serious side effects call for prompt medical attention:
- Bradycardia, or sluggish heartbeat: Heart rate reduction is a possible side effect of ozanimod, particularly after the initial dose. It is advised to monitor while starting treatment.
- Elevations of Liver Enzymes: A rise in liver transaminases could be a sign of liver damage. It is recommended to have routine liver function testing to identify hepatotoxicity early.
- Macular edema: Changes in vision may result from swelling in the central retina. Patients should report any changes in vision as soon as possible, and routine ophthalmologic examinations are advised.
Take Care
Some medical histories and current drug regimens require prudence:
- Previous Health Issues: Ozanimod should be used cautiously in patients with a history of heart problems (such as myocardial infarction, unstable angina), liver illness, or uveitis (inflammation of the eyes). Prior to starting therapy, a comprehensive medical checkup is necessary.
- Drug Interactions: Because of the possibility of a hypertensive crisis, ozonimod and monoamine oxidase inhibitors (MAOIs) shouldn’t be taken together. When combined with other medications that impact immunological response or heart rate, caution is also recommended.
- Monitoring Requirements: Patients should undergo baseline assessments, such as electrocardiograms (ECGs), liver function tests, and eye exams, prior to commencing ozanimod. Live vaccines should not be administered during and for up to three months following treatment, therefore lymphocyte numbers should be checked and immunization records updated.
Safety Measures
- Infection Risk: Immune modulation makes a person more vulnerable to infections. Patients should be on the lookout for infection symptoms and should consult a doctor if they do.
- Pregnancy and Lactation: Ozanimod may cause fetal damage. For three months following treatment termination and throughout therapy, women who are capable of becoming pregnant should use an effective form of contraception. When taking ozanimod, breastfeeding is not recommended.
By being aware of these risks and side effects, patients and healthcare professionals may make well-informed decisions and make sure that ozanimod’s advantages outweigh its disadvantages. Ensuring safe and successful therapy requires regular monitoring and open communication with healthcare experts.
Conclusion
Ozanimod (Zeposia) stands as a promising advancement in the management of relapsing multiple sclerosis (MS) and moderately to severely active ulcerative colitis (UC). Its unique mechanism of selectively modulating sphingosine 1-phosphate (S1P) receptors allows for targeted immune suppression, reducing disease activity while minimizing systemic side effects. The convenience of once-daily oral administration enhances patient adherence, offering a significant advantage over traditional injectable therapies.
Ozanimod has been shown in clinical trials to be effective in lowering relapse rates, postponing the progression of disability in MS patients, and causing and sustaining remission in UC patients. These advantages help patients live better lives by giving them the confidence and ability to handle their ailments more skillfully.
It is imperative that patients who are thinking about ozanimod as a therapeutic option have frank discussions with medical experts. Patients who are aware of the possible advantages, risks, and required monitoring can be better equipped to make decisions that support their overall health. In order to optimize treatment results and guarantee safety, routine examinations and strict adherence to recommended protocols are essential.
References
- Sandborn, W. J., Feagan, B. G., D’Haens, G., et al. (2020). Ozanimod as induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine, 383(14), 1280–1291.
- Cohen, J. A., Comi, G., Selmaj, K., et al. (2019). Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): A multicentre, randomised, minimum 12-month, phase 3 trial. The Lancet Neurology, 18(11), 1009–1020.
- Scott, F. L., Clemons, B., Brooks, J., et al. (2016). Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor modulator with autoimmune disease-modifying activity. British Journal of Pharmacology, 173(11), 1778–1792.
- Cohen, J. A., Comi, G., Selmaj, K., et al. (2020). Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): A multicentre, randomised, phase 3 trial. The Lancet Neurology, 19(11), 991–1001.
- Comi, G., Kappos, L., Selmaj, K. W., et al. (2019). Efficacy and safety of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): A multicentre, randomised, 24-month, phase 3 trial. The Lancet Neurology, 18(11), 1021–1033.




