Peptide Drug Complexes (PDCs): Advancements, Mechanisms, and Clinical Applications
Abstract
Peptide Drug Complexes (PDCs) are an emerging prodrug strategy in targeted drug delivery systems. They consist of three components: peptides, linkers, and drugs. The mechanism of action varies depending on the types of peptides and linkers. Firstly, peptides enter cells by recognizing specific receptors on the cell surface. Subsequently, under certain stimuli, the linkers are cleaved, releasing the drug and exerting its therapeutic effects. This prodrug strategy has the potential to enhance drug targeting and reduce toxic side effects on other cells.
Compared to the strategy of constructing ADCs (Antibody-Drug Conjugates), PDCs (Peptide Drug Complexes) have unique advantages:
(1) Peptides have smaller molecular size and higher drug-loading capacity, which enables easier penetration of the tumor stroma and entry into tumor cells. (2) Peptides are highly biodegradable and do not induce any immunogenic reactions in the body. (3) Certain targeted peptides can alter the cellular entry mechanism of drugs, overcoming drug resistance in tumor cells and effectively killing resistant tumors. (4) The short peptide nature of PDCs makes them structurally flexible and easy to modify and conjugate. (5) They can be linked with various drugs such as chemicals, proteins, and peptide-based drugs to prepare targeted drugs, significantly reducing off-target toxicity.The production process of peptide fragments is simple and amenable to scaling up.
Therefore, PDCs have great potential in the development of targeted delivery systems.
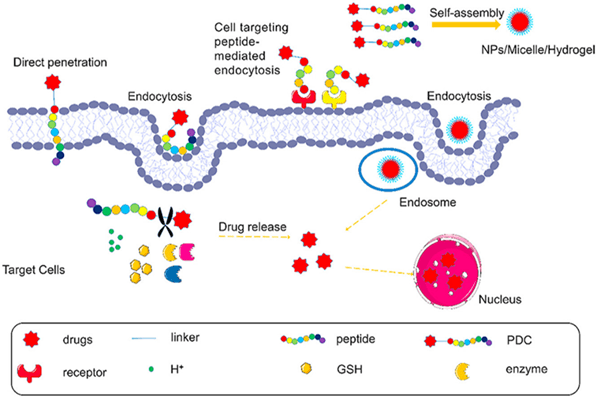
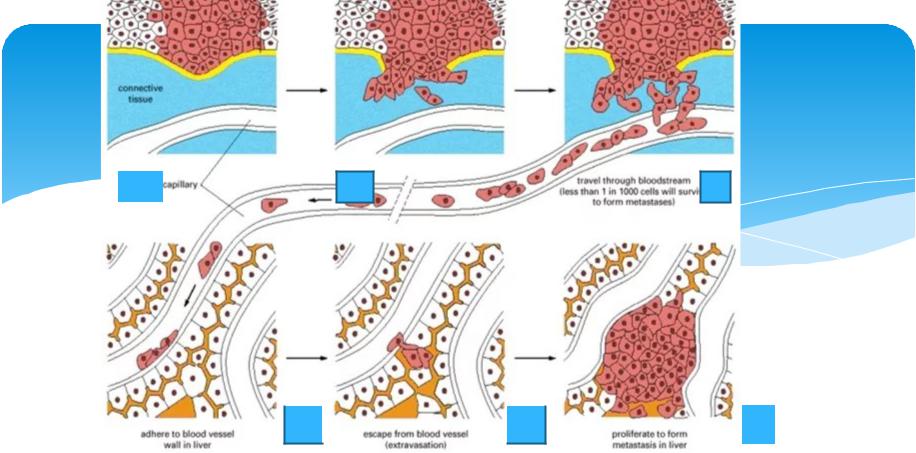
Fig 1. Peptide-drug conjugates (PDCs) enter the cell by direct penetration or mediated endocytosis of peptide, and then the linker is stimulated to cleave responsively, resulting in the release of the drug [1].
The types and mechanisms of Peptides
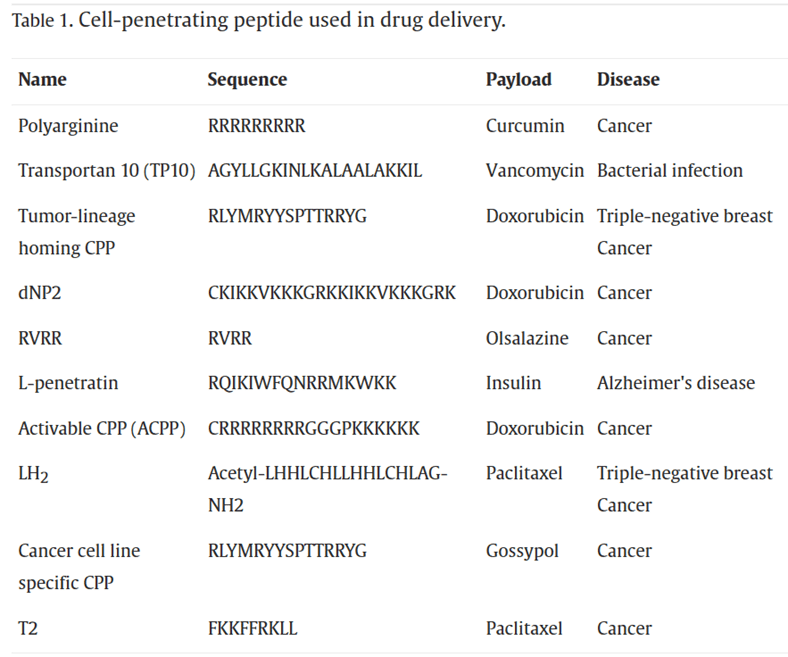
Peptides are important components of PDCs. Peptides can be categorized based on their functions into Cell-Penetrating Peptides (CPPs), Cell-Targeting Peptides (CPTs), Self-Assembling Peptides (SAPs), and Responsive Peptides. Cell-Penetrating Peptides (CPPs) refer to small, short peptide molecules that can enter cells without disrupting their membrane integrity. They typically consist of 5-30 amino acids. CPPs contain abundant basic amino acids such as arginine and lysine, which carry a positive charge in physiological environments. In recent years, CPPs have been widely used in drug delivery systems. The mechanisms of CPP membrane penetration can be categorized into two classes: endocytosis and direct translocation.
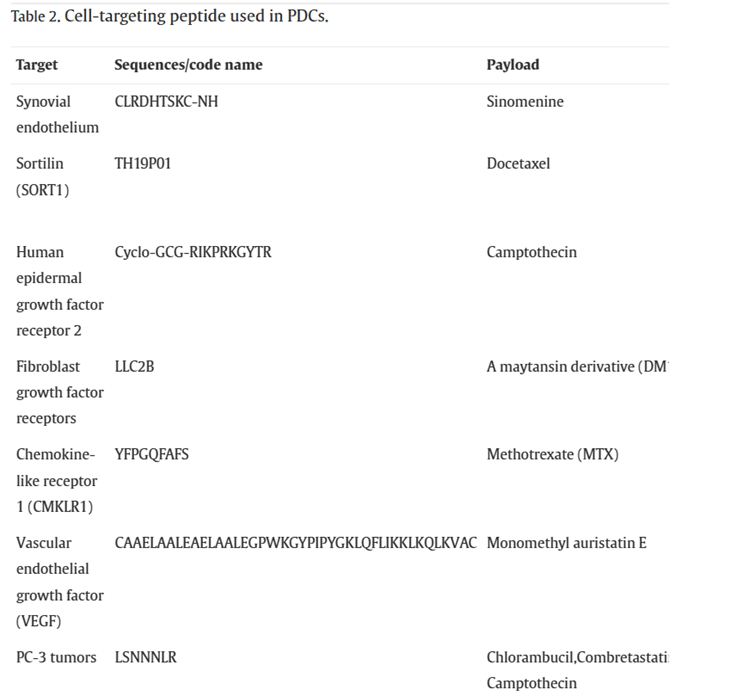
Cell-Targeting Peptides (CPTs) are defined as peptides that specifically bind to cells or tissues. Generally, there are two types of targeting delivery systems: passive targeting and active targeting. Passive targeting refers to the passive accumulation of drugs in diseased tissues due to the properties of the delivery system itself (size, shape, charge, etc.) or the characteristics of the target tissue (poor vascularization, inadequate lymphatic drainage, etc.) as they circulate in the bloodstream. Active targeting involves actively delivering drugs to diseased tissues by recognizing receptors or proteins that are specifically expressed in the target tissue and utilizing receptor-ligand interactions. Most CPTs achieve drug delivery through active targeting mechanisms.
Self-Assembling Peptides (SAPs) refer to peptides that spontaneously form one or multiple ordered structures from complex mixtures through non-covalent interactions, including van der Waals forces, electrostatic interactions, hydrogen bonding, and stacking interactions, without the need for external stimuli. Amino acids serve as the basic building blocks of peptides, and the type and arrangement of amino acids form the foundation of the self-assembly ability of peptides. Furthermore, the key to designing self-assembling peptides lies in utilizing various intermolecular interactions, including hydrogen bonding, electrostatic interactions, π-π stacking, van der Waals forces, metal ligand complexes, and entropy. Peptides with self-assembly capabilities tend to exhibit amphiphilicity, aromaticity, charge dispersity, and alternating arrangement of symmetric charges. Compared to regular peptides, SAPs have significant advantages such as biocompatibility, biodegradability, and multifunctionality.
The Classification of Linkers
As the bridge between drugs and peptides, linkers play a crucial role in determining the circulation time and stability of PDCs in the body. An ideal linker should maintain stability during circulation, avoiding premature drug release. However, it should also facilitate the rapid and efficient release of the drug upon reaching the targeted diseased tissue. Additionally, the linker should not interfere with the affinity of the peptide for its receptors or the pharmacological activity of the drug.
The hydrophobicity of the linker should not be too strong to prevent the aggregation of PDCs due to hydrophobic interactions. Aggregation can lead to poor stability in the body, reduced therapeutic efficacy, as well as increased systemic toxicity and immunogenicity. Based on the drug release mechanism, linkers can be mainly categorized into non-cleavable linkers and cleavable linkers. Cleavable linkers can further be classified into pH-sensitive linkers, redox-sensitive linkers, and enzyme-sensitive linkers.
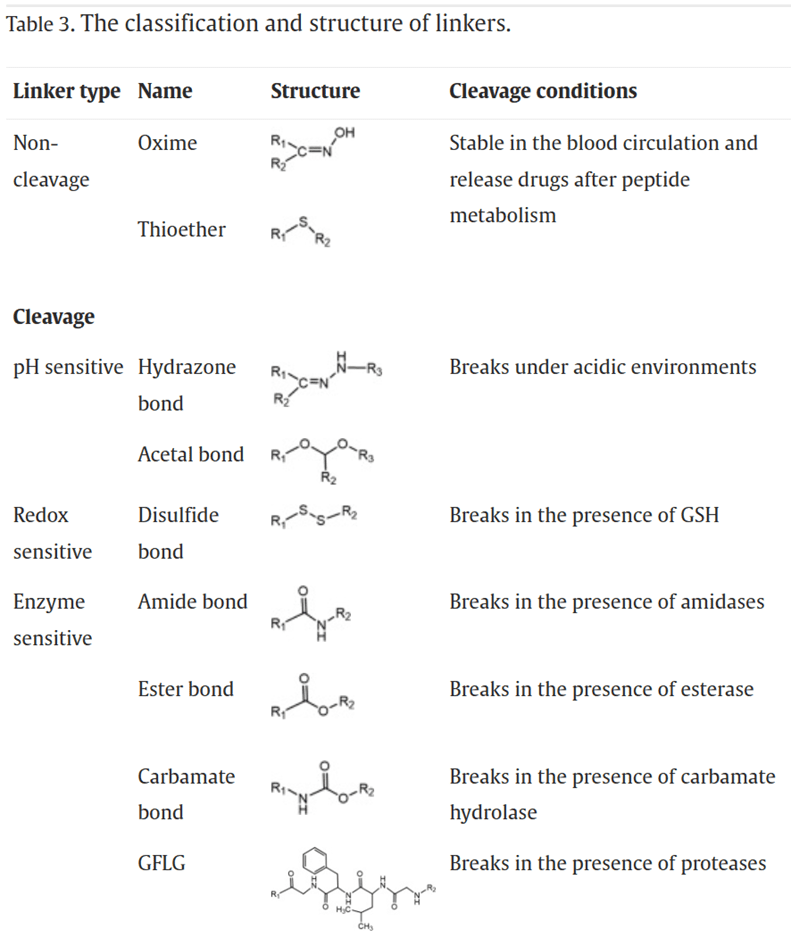
Drugs for Constructing PDCs
The effective payload of PDCs consists of drugs that have cytotoxic or therapeutic effects. However, these drugs often have limitations such as low water solubility, poor selectivity, short half-life, and low stability, which restrict their clinical application. Drugs delivered using the PDC strategy require feasible binding sites, no pharmacological activity upon binding, clear therapeutic mechanisms, and strong pharmacological activity when released in diseased tissues. When coupled with peptides, the drugs can enhance their solubility, promote selectivity, prolong circulation time in the body, optimize bioavailability, and prevent side effects and toxicity to other tissues. Drugs used for PDC construction can be classified into chemical drugs, protein drugs, and peptide drugs.
Chemical drugs include doxorubicin, paclitaxel, camptothecin, methotrexate, and cisplatin. Protein drugs include interferons and tumor necrosis factor, which achieve effective anti-tumor therapy by inducing cell apoptosis and inhibiting intracellular protein synthesis. However, protein drugs have disadvantages such as poor in vivo stability, lack of targeting specificity, and low bioavailability. Therefore, the combination of peptides and protein drugs to form PDCs has emerged as a new approach to address these issues. Peptide drugs have become a hot topic in recent years for new drug development.
Peptide drugs have significant advantages compared to traditional chemical drugs and protein drugs: (1) Peptide drugs generally exhibit higher activity. (2) Compared to protein drugs, peptide drugs have smaller molecular weight, making them easier to synthesize artificially and modify structurally. (3) Peptide drug synthesis is efficient, and with advancements in technology, solid-phase peptide synthesis has become simple, highly automated, and easy to control. (4) Peptide drugs have fewer side effects. Most peptide drugs have high homology with human sequences, small molecular weight, and are non-immunogenic, meaning they do not induce an immune response.
However, peptide drugs also have notable disadvantages. Unlike chemical drugs and protein drugs, peptide drugs have unstable physicochemical properties, are prone to oxidation and hydrolysis, and tend to aggregate. Additionally, peptide drugs have a short half-life and fast clearance rate, making it challenging for them to penetrate cell membranes.
PDCs for the treatment of different diseases
PDCs can be used for the treatment of inflammation, such as naproxen is a nonsteroidal anti-inflammatory drug that exerts its anti-inflammatory effects by inhibiting prostaglandin synthesis. However, due to its lack of selectivity, it can cause gastrointestinal adverse reactions. In order to enhance the selectivity of naproxen, Moreira et al. constructed naproxen-dipeptide complexes and found that they readily form nanostructured fibrous supramolecular hydrogels, which may represent an optimal strategy for treating inflammation.
In addition, PDC drugs are widely used for the treatment of bacterial infectious diseases. Effective and safe treatment of bacterial infections is a major challenge in modern medicine. This is largely due to the limitations of current antibacterial drugs in terms of host toxicity, effective delivery, and the emergence of antibiotic resistance. To address these issues, Brankiewicz et al. covalently linked fluconazole with a cell-penetrating peptide (CPT) to form a PDC. Compared to free fluconazole, the PDC demonstrated higher efficacy in killing Candida albicans, a type of fungus causing yeast infections.
In addition, PDC drugs are widely used for the treatment of bacterial infectious diseases. Effective and safe treatment of bacterial infections is a major challenge in modern medicine. This is largely due to the limitations of current antibacterial drugs in terms of host toxicity, effective delivery, and the emergence of antibiotic resistance. To address these issues, Brankiewicz et al. covalently linked fluconazole with a cell-penetrating peptide (CPT) to form a PDC. Compared to free fluconazole, the PDC demonstrated higher efficacy in killing Candida albicans, a type of fungus causing yeast infections.
In addition, PDCs can also be applied to delivery systems for the treatment of drugs such as pain relief, malaria, and diabetes. As long as the drugs meet the requirements for PDC construction, it is believed that in the future, this prodrug strategy can be used to achieve the treatment of more diseases.
Prospects of clinical application of PDCs drugs
Although PDCs have great potential for application, the field of PDCs is still in a relatively early stage of development. Currently, there are only two approved PDC drugs on the global market. One is Lutathera, developed by a subsidiary of Novartis, which received FDA approval in January 2018 for the treatment of somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors. The other is Pepaxto (Melflufen), developed by Oncopeptides, which received accelerated FDA approval in February 2020 for the treatment of multiple myeloma.
Summary and Outlook
PDC drugs are primarily delivered to specific sites (actively or passively targeted) through peptide targeting. They are then effectively delivered into cells through the membrane penetration of cell-penetrating peptides or receptor-mediated translocation. Finally, under environmental stimuli or enzyme action, the specific linkages are cleaved to release the payload, thereby exerting the therapeutic effect.
Despite the numerous unique advantages of PDCs, there are still many challenges and obstacles in their clinical translation, as evident from the limited number of PDC drugs available on the market. Firstly, in terms of safety, peptides derived from natural amino acids are generally water-soluble, biodegradable, and biocompatible, without adverse reactions. However, it remains unclear whether the biocompatibility and biodegradability of peptides are preserved after conjugation with drugs.
Secondly, considering stability, peptides have relatively short biological half-lives, which limits the distribution and circulation time of PDCs in the body. The time for drugs to enter the target tissue cells before peptide degradation is limited. Although there are various methods to extend the biological half-life of peptides, PDC drugs, due to their small size, are prone to renal clearance. Therefore, it is recommended to combine the prodrug strategy of PDCs with carrier materials to form a delivery system of suitable size and higher stability.
Thirdly, considering efficacy, the linkers in PDCs require specific environmental conditions (such as temperature, pH, redox potential, enzymes, etc.) to be cleaved and release the drugs. Sometimes the drugs may not be released as prototype drugs or may not be released at all. Additionally, it is challenging to determine the release rate and efficiency of drugs in target cells. Therefore, appropriate methods are needed to demonstrate the therapeutic efficacy of PDC drugs.
Lastly, in terms of administration, due to the presence of gastrointestinal proteases, similar to protein-based drugs, PDC drugs currently cannot be administered orally and are limited to invasive injection routes, which restricts their clinical application. To overcome this limitation, it is recommended to design oral delivery systems for large molecular drugs and utilize appropriate carriers or structural modifications to prevent enzymatic degradation in the gastrointestinal system and ensure the absorption of PDCs into the bloodstream.
The development of efficient PDCs requires the design and synthesis of multifunctional peptides. We believe that with advancements and maturity in peptide screening and synthesis technologies, more multifunctional peptides will be explored. Intelligent nano drug delivery strategies based on PDCs are also expected to be widely applied in clinical settings in the future.
Reference
- Gong, L., Zhao, H., Liu, Y., Wu, H., Liu, C., Chang, S., … & Huang, W. (2023). Research advances in peptide‒drug conjugates. Acta Pharmaceutica Sinica B.
- Hennrich, U., & Kopka, K. (2019). Lutathera®: the first FDA-and EMA-approved radiopharmaceutical for peptide receptor radionuclide therapy. Pharmaceuticals, 12(3), 114.
- Mateos, M. V., Bladé, J., Bringhen, S., Ocio, E. M., Efebera, Y., Pour, L., … & Richardson, P. G. (2020). Melflufen: a peptide–drug conjugate for the treatment of multiple myeloma. Journal of clinical medicine, 9(10), 3120.
- Kumthekar, P., Tang, S. C., Brenner, A. J., Kesari, S., Piccioni, D. E., Anders, C., … & Ibrahim, N. (2020). ANG1005, a Brain-Penetrating Peptide–Drug Conjugate, Shows Activity in Patients with Breast Cancer with Leptomeningeal Carcinomatosis and Recurrent Brain MetastasesANG1005 for Leptomeningeal and CNS Metastases. Clinical Cancer Research, 26(12), 2789-2799.