Peptide-drug conjugates (PDCs) for cancer treatment
Abstract
Oncology drug discovery has been a relentless pursuit, evolving through various phases and shifting paradigies over the years. Traditionally, the search for cancer therapeutics has oscillated between small molecule drugs, typically below 500 Daltons, and biologics such as antibodies, which are substantially larger, often around or above 45,000 Daltons. The development of these agents has typically been a response to the pressing need for treatments that can specifically target and destroy cancer cells with minimal damage to normal tissues.
In this complex landscape, peptides, defined by the FDA as polymers composed of less than 40 amino acids, have emerged as a promising platform. They occupy a unique niche in the molecular weight spectrum, ranging from 500 to 5000 Daltons, and offer several advantages over both small molecules and larger biologic therapies. Peptides are recognized for their specificity and high binding affinity, which can be fine-tuned for targeted therapeutic applications. Moreover, they are generally less immunogenic and potentially cheaper to synthesize compared to larger protein-based therapeutics. Despite these advantages, peptides face challenges, including limited oral bioavailability and rapid degradation by proteases in the body, which have historically slowed their clinical development.
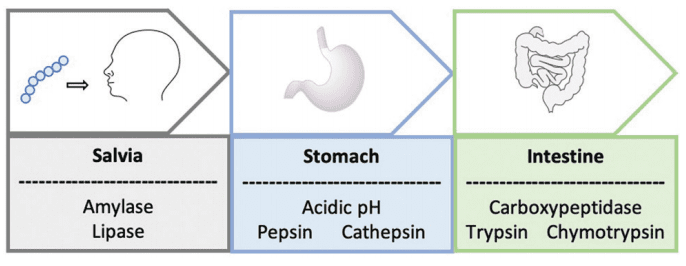
Figure. 1 A schematic to represent the journey of an orally administrated peptide through the gastrointestinal tract
Peptide-drug conjugates (PDCs) represent a novel class of targeted therapeutics designed to address some of these limitations. A PDC typically consists of a peptide linked to a drug or cytotoxic agent, forming a conjugate that combines the targeting capabilities of peptides with the potent therapeutic effects of the drug payload. This strategy leverages the peptides’ ability to home in on specific receptors or cellular targets overexpressed in cancer cells, ensuring that the drug is delivered directly to the tumor site. This targeted approach not only enhances the efficacy of the therapeutic agent but also reduces the off-target toxicity associated with more traditional forms of chemotherapy.
The role of PDCs in oncology is transformative, providing a means to increase tumor penetration and selectivity of cancer drugs. The design of PDCs involves sophisticated chemical engineering to optimize the stability of the peptide in circulation and ensure controlled release of the drug at the target site. Successful examples of PDCs in the market and various stages of clinical trials illustrate the potential of this technology to significantly impact cancer treatment paradigms.
Overall, the integration of peptides into oncology drug development represents a significant advancement, bridging the gap between small molecules and biologics. As research continues to unravel the complexities of cancer biology, peptides are poised to play a crucial role in the next generation of oncology therapeutics, offering a promising pathway for treatments that are not only effective but also safer and more specific.
Advantages and Challenges of Peptides as Therapeutics
Peptides have increasingly become a focal point in the realm of drug design and therapeutic strategies due to their unique properties and potential advantages. One of the most significant benefits of peptides in drug development is their remarkable specificity and high affinity for target sites, attributes that are crucial in the precise targeting of disease markers. Compared to small molecules, peptides can engage larger and more complex surfaces on target proteins, often leading to more effective interactions with biological targets that are typically considered “undruggable” by conventional drugs. This capability allows for the design of therapeutics that can intervene in intricate cellular processes with minimal disruption to other cellular functions, reducing side effects and improving patient outcomes.
Despite these promising features, the therapeutic use of peptides is not without challenges. One major hurdle is their stability. Peptides are prone to rapid degradation by proteolytic enzymes in the bloodstream, which can severely limit their half-life and, consequently, their therapeutic efficacy. To overcome this, significant efforts have been directed toward modifying peptides chemically, such as by pegylation or through the incorporation of unnatural amino acids, to enhance their stability and resistance to enzymatic breakdown.
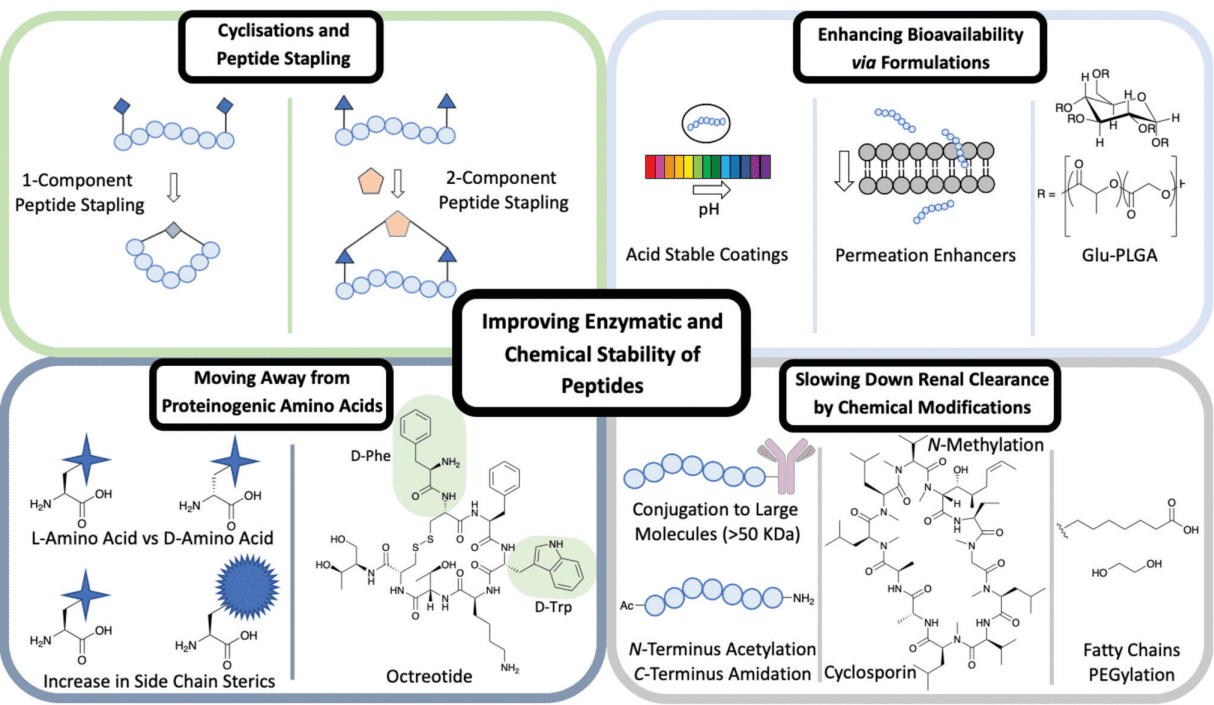
Figure. 2 Different methods used to improve the enzymatic and chemical stability of peptides including: cyclisations and peptide stapling, formulations, moving away from proteinogenic amino acids and chemical modifications
Another significant challenge is the limited oral bioavailability of peptides, which stems from their size and structural properties that render them vulnerable to digestive enzymes and poor absorption in the gastrointestinal tract. Most peptide drugs thus require alternative routes of administration, such as injection, which can limit patient compliance and increase the complexity of treatment regimens.
Despite these challenges, the market for peptide therapeutics has seen substantial growth and success. Over 100 peptide drugs have gained approval across various global markets, and they are now used to treat a wide range of conditions, including cancers, diabetes, and infectious diseases. The financial landscape reflects this success, with the peptide therapeutic market valued at several billion dollars annually, and projections indicating continued growth. This commercial success is supported by advancements in peptide synthesis technologies, which have reduced costs and improved the scalability of peptide production.
The future for peptide therapeutics continues to be promising as ongoing research addresses their inherent challenges. Innovations in peptide drug formulation and delivery are particularly active areas of development, aiming to enhance the stability, bioavailability, and overall clinical efficacy of peptide-based drugs. As these efforts bear fruit, peptides are expected to play an increasingly prominent role in the pharmaceutical landscape, offering more targeted and less toxic alternatives for treatment across a broad spectrum of diseases.
Peptide-Drug Conjugates (PDCs)
Peptide-drug conjugates (PDCs) are sophisticated therapeutic entities designed to harness the targeting capability of peptides coupled with the potent activity of traditional pharmaceutical agents. Structurally, a PDC consists of three primary components: a peptide that targets specific cells or receptors, a cytotoxic drug that serves as the payload, and a linker that holds these two components together. The linker not only stabilizes the construct but also ensures that the drug is released in a controlled manner once the target site is reached. This strategic assembly aims to combine the best features of peptides and drugs, optimizing the delivery and efficacy of the treatment while minimizing side effects.
The primary mechanism of action for PDCs revolves around targeted drug delivery, which is pivotal in cancer therapy. The targeting peptides specifically bind to receptors or antigens that are overexpressed on the surface of cancer cells, facilitating the direct delivery of the drug to the tumor. This selective approach helps to maximize the therapeutic impact on the tumor while sparing healthy cells, thus reducing the systemic toxicity associated with conventional chemotherapy. After binding to the target, PDCs are often internalized by the cancer cells, and once inside, the linker is cleaved either enzymatically or through changes in the cellular environment (like pH), releasing the cytotoxic drug to exert its therapeutic effect.

Figure. 5 A schematic of a peptide–drug conjugate construct consisting of a homing peptide, linker and payload. The structure of 177Lu-dotatate an FDA approved peptide–drug conjugate
There are several notable examples of PDCs that have reached the market or are in various stages of clinical trials, highlighting the potential and efficacy of this approach. One such example is 177Lu-dotatate (Lutathera®), which targets somatostatin receptors that are overexpressed in certain types of neuroendocrine tumors. This PDC uses a radiolabeled peptide to deliver a lethal dose of radiation directly to the tumor cells. Another example in clinical development is Melflufen (Melphalan flufenamide), designed for multiple myeloma treatment. It utilizes a peptide-drug conjugate strategy where the peptide carrier enhances the delivery of the alkylating drug to cells with high aminopeptidase activity, common in myeloma cells.
These examples underscore the growing interest and potential of PDCs as a next-generation approach in cancer therapy. With ongoing advancements in peptide chemistry, linker technology, and a better understanding of tumor biology, the development of PDCs continues to expand, offering new hope for more effective and less toxic cancer treatments. As research progresses, the role of PDCs is set to become increasingly significant in the pharmaceutical landscape, providing a powerful tool against cancer and possibly other diseases where targeted therapy offers a distinct advantage.
Design and Development of PDCs
The design and development of peptide-drug conjugates (PDCs) involve intricate chemical engineering and strategic planning to ensure that these therapeutics are not only effective in targeting and destroying cancer cells but also stable enough to survive in the biological environment until they reach their target. This section details the innovative strategies used in PDC design, focusing on enhancing peptide stability, the overall design process, and the crucial role of understanding the mechanism of action.
Chemical and Nanomaterial Strategies to Enhance Peptide Stability:
To improve the stability of peptides within PDCs, researchers employ various chemical modifications and nanomaterial technologies. Chemical strategies include the use of unnatural amino acids to prevent degradation by natural enzymes, cyclization of the peptide backbone to enhance resistance to enzymatic cleavage, and the incorporation of protective groups such as PEGylation, which can shield peptides from rapid renal clearance and proteolytic breakdown. On the nanomaterial front, researchers have developed innovative approaches such as encapsulating peptides in biocompatible nanoparticles to protect them from premature degradation and to facilitate targeted delivery through enhanced permeability and retention (EPR) effect in tumors. These nanoparticles can also be designed to respond to specific stimuli within the tumor microenvironment, such as pH or enzyme concentration, triggering the release of the peptide and the drug at the site of the tumor.
Details on the Design Process of PDCs:
The design process of PDCs begins with the selection of a target-specific peptide that can recognize and bind to receptors overexpressed on cancer cells. This is followed by the choice of a potent cytotoxic drug that can effectively kill cancer cells once delivered by the peptide. The third component, the linker, is crucial as it connects the peptide to the drug. The linker must be stable enough to prevent premature release of the drug during circulation but sensitive enough to release the drug upon reaching the target. This requires careful consideration of the linker chemistry, taking into account factors such as the cleavage mechanism (e.g., enzymatic, pH-sensitive) that will activate drug release. The entire construct must be optimized for biocompatibility, solubility, and the ability to evade the immune system while maintaining efficacy.
Importance of Understanding the Mechanism of Action for Effective PDC Design:
A thorough understanding of the mechanism of action is essential for the effective design of PDCs. This includes knowledge of how the peptide interacts with its target, how the drug exerts its cytotoxic effects, and how the linker releases the drug at the appropriate time and place. Understanding these mechanisms allows researchers to fine-tune each component of the PDC to optimize its therapeutic efficacy and safety. For example, if a peptide is known to internalize into cancer cells upon binding, a cleavable linker might be used to release the drug inside the cells. Alternatively, if the target receptor does not internalize, a non-cleavable linker might be preferable to release the drug outside the cells, allowing it to diffuse into the tumor tissue.
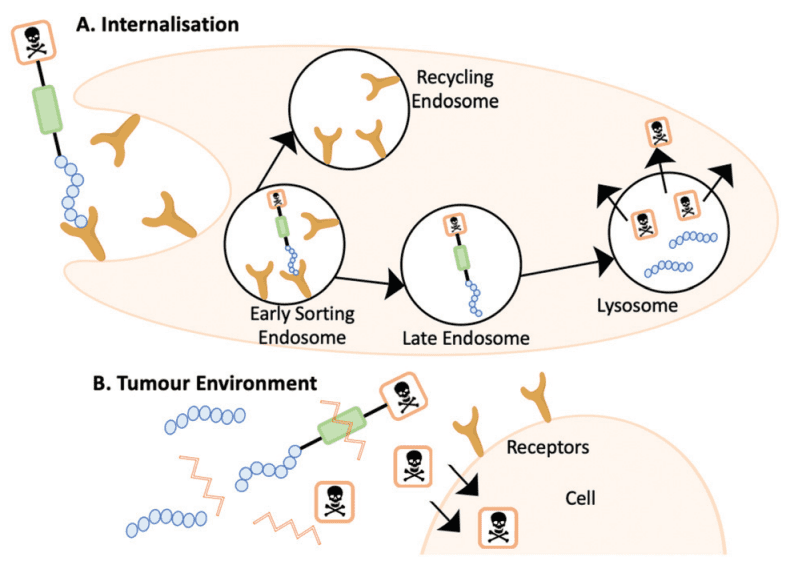
Figure. 4 (A) The internalisation mechanism of action where the PDC is cleaved within the lysosome. (B) The PDC is cleaved outside of the cell and the cytotoxin diffuses into the cell
Overall, the design and development of PDCs require a multi-disciplinary approach that integrates peptide chemistry, drug pharmacology, and materials science to create a new generation of cancer therapeutics that are both effective and safe. As our understanding of tumor biology and chemistry advances, so too will the sophistication and success of PDCs in clinical settings.
Clinical Trials and Market Status
The field of peptide-drug conjugates (PDCs) is rapidly advancing, with several promising candidates currently undergoing clinical trials. These developments are not only pivotal for medical science but also have significant implications for the pharmaceutical market. This section provides an overview of PDCs in clinical trials and an analysis of their market potential and economic impact.
Overview of PDCs Undergoing Clinical Trials:
Clinical trials for PDCs span a variety of phases, each aiming to explore the efficacy, safety, and optimal dosing of these innovative therapeutics. Many of these trials target prevalent and challenging cancers, demonstrating the potential of PDCs to address unmet needs in oncology. For instance, 177Lu-dotatate, a well-known PDC, has shown significant promise in treating neuroendocrine tumors and has gained FDA approval, highlighting the potential for other PDCs in the pipeline. Other notable examples include Melflufen, targeting multiple myeloma, and various other candidates focusing on solid tumors and hematological malignancies. These trials are critical not only for evaluating the therapeutic potential of PDCs but also for understanding their pharmacokinetics and pharmacodynamics, which are essential for successful drug development.
Market Analysis and the Economic Potential of PDCs:
The market for PDCs is expected to grow significantly in the coming years, driven by the increasing incidence of cancer and the need for more effective and targeted treatment options. The specificity of PDCs in targeting tumor cells while sparing healthy tissues offers a compelling advantage over traditional chemotherapy, which is often associated with severe side effects. This specificity is expected to lead to better patient outcomes and compliance, driving demand for PDC-based therapies.
Financially, the global market for PDCs is part of the broader oncology drugs market, which is projected to reach substantial values by the end of the decade. The growth of the PDC segment is supported by increased investment in research and development by pharmaceutical companies, driven by the potential for high returns on investment associated with successful cancer drugs. Additionally, the ability of PDCs to potentially extend lives and reduce treatment-related complications presents an opportunity for healthcare systems to save on long-term patient care costs, making these drugs economically attractive despite their likely high initial costs.
The successful commercialization of PDCs hinges on several factors, including the demonstration of clear benefits over existing therapies in terms of efficacy and safety, favorable regulatory outcomes, and the establishment of strong commercial strategies to reach key markets. Pharmaceutical companies are increasingly recognizing the value of investing in targeted therapies such as PDCs, and partnerships and collaborations in this field are becoming more common as companies seek to leverage each other’s strengths in drug development and marketing.
Future Prospects
The field of peptide-drug conjugates (PDCs) is on the brink of significant transformative advances, promising to redefine therapeutic strategies in oncology. The potential for innovation in this domain is vast, driven by emerging scientific insights and technological advancements.
Research into PDCs continues to evolve, focusing on enhancing efficacy, reducing toxicity, and expanding the range of treatable conditions. Innovations include the development of more stable and selective linkers that can respond to the unique microenvironment of different tumors, improving the controlled release of cytotoxic agents. Additionally, the integration of novel imaging and diagnostic capabilities into PDCs—creating theranostic agents—promises to enhance the precision of oncology therapies, allowing for real-time monitoring of treatment efficacy and adjustments.
As the understanding of cancer biology deepens, new molecular targets are continually being identified. Future PDCs are expected to exploit less traditional targets, such as cancer stem cells and the tumor microenvironment, including immune cells, to prevent tumor growth and recurrence effectively. Moreover, multi-targeted PDCs, which can engage several pathways simultaneously, are being considered to overcome resistance mechanisms and improve clinical outcomes. The exploration of combination therapies, pairing PDCs with other treatments like immunotherapy or radiotherapy, is another promising avenue that could synergistically enhance therapeutic effects and combat complex cancer mechanisms.
Conclusion
Peptides hold a unique position in oncology drug development due to their specificity, efficiency, and versatility. As targeted therapeutic agents, they offer the potential to minimize side effects typically associated with conventional cancer treatments, providing a higher quality of life during therapy. The ongoing advancements in peptide engineering, conjugate design, and drug delivery technologies continue to unlock new opportunities for peptides in oncology, making them integral components of future cancer therapies.
The evolution of peptide-drug conjugates marks a significant milestone in the pursuit of more precise and personalized cancer treatment options. PDCs exemplify how targeted therapeutic strategies can dramatically improve treatment outcomes by effectively combining the targeting capabilities of peptides with the potent effects of drugs. As this field progresses, PDCs are expected to lead to a broader paradigm shift in oncology treatment practices, emphasizing targeted, personalized medicine over traditional approaches.
The future landscape of cancer therapy, enriched by PDCs, looks promising. With each research breakthrough and successful clinical trial, the potential of PDCs to provide safer, more effective cancer treatments becomes increasingly tangible. This ongoing evolution will undoubtedly continue to impact the lives of patients worldwide, offering hope through more efficient and targeted therapeutic options that aim to not only treat but ultimately cure cancer.