Recent Research on Chemokines and Chemokine Receptors
Abstract
Chemokines and chemokine receptors play key roles in cancer progression, metastasis, and therapy. Chemokines attract immune cells to tumor sites, while chemokine receptors facilitate their migration into tumors. Targeting chemokine receptors with small molecule inhibitors is a promising therapeutic strategy for many types of cancer, including breast cancer. However, challenges remain in the development of effective chemokine-based therapies, including the identification of novel chemokine receptors and the development of better predictive biomarkers. This article describes the role of chemokines and chemokine receptors and potential research directions.
Introduction to the Tumor Microenvironment
The tumor microenvironment (TME) comprises cellular and non-cellular components surrounding a tumor, such as immune cells, stromal cells, extracellular matrix, and signaling molecules. It plays a vital role in tumor growth, invasion, and metastasis, as well as in the immune response to cancer.
The TME is characterized by immunosuppressive cells, such as myeloid-derived suppressor cells and regulatory T cells, that hinder anti-tumor immune responses. Additionally, the TME secretes signaling molecules, including cytokines and chemokines, that govern immune cell recruitment and activity.
Chemokines are a group of small signaling proteins that play a crucial role in immune cell recruitment to the TME. They interact with chemokine receptors on immune cells to induce directional migration toward the tumor site. The expression of chemokines and chemokine receptors can be dysregulated in the TME, leading to altered immune cell infiltration and activity.
Comprehending the intricate interplay between tumor cells, immune cells, and the TME is pivotal in creating efficacious cancer treatments. Targeting chemokines and chemokine receptors is a promising avenue for cancer immunotherapy, as these molecules can regulate immune cell recruitment and activity in the TME. Modulating these molecules could enhance anti-tumor immune responses and boost patient outcomes.
The Role of Chemokines in Immune Cell Recruitment to Tumors
Chemokines play a crucial role in recruiting immune cells to tumors, which is vital for the anti-tumor immune response. These small cytokines signal through chemokine receptors on immune cells’ surfaces, directing them to specific locations within the TME. Chemokines act as chemoattractants for immune cells like T cells, B cells, natural killer (NK) cells, dendritic cells (DCs), and macrophages, facilitating their migration toward the tumor site.
Chemokines and chemokine receptors expression can differ among various types of cancer in the tumor microenvironment (TME). While some tumors can overexpress specific chemokines, which can potentially promote immune cell recruitment, others may exhibit chemokine receptors expression that could impede immune cell migration or activation. This highlights the complexity and dynamics of the TME in cancer progression and therapy.
Chemokines and their receptors play a critical role in the immune surveillance of cancer, as they regulate the trafficking of immune cells from circulation into the TME. Immune cell infiltration is a critical step in generating an effective anti-tumor immune response. However, tumors can develop various mechanisms to evade immune surveillance, such as by downregulating the expression of chemokines or by recruiting immunosuppressive cells, like myeloid-derived suppressor cells and regulatory T cells, that hinder the activity of immune cells.
Targeting chemokines and their receptors has emerged as a promising strategy to enhance immune cell recruitment and activation in the TME. By modulating chemokine signaling, it may be possible to promote the infiltration of effector immune cells while reducing the recruitment of immunosuppressive cells, thus promoting an effective anti-tumor immune response.
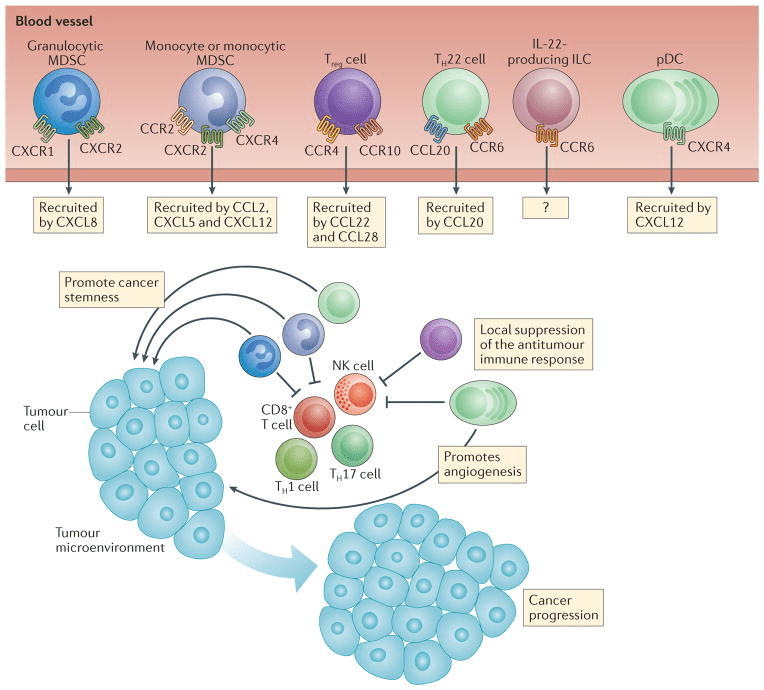
Figure 1. Pro-tumor effects of chemokines. [6]
Introduction to chemokine receptors and their roles in disease pathogenesis
Chemokine receptors belong to a family of G protein-coupled receptors that participate in various physiological and pathological processes, such as inflammation, immune cell migration, and cancer progression.
Chemokine receptors are present in a range of cell types, including immune cells, stromal cells, and cancer cells, and they bind with chemokines, which are small signaling proteins released by cells in response to inflammation. This binding is responsible for the recruitment of immune cells to sites of inflammation and tumors, where they can either promote or suppress immune responses. Dysregulated chemokine receptor expression or activity is linked to various diseases, such as cancer, inflammatory disorders, and viral infections. As a result, targeting chemokine receptors has emerged as a promising approach for the development of novel therapies for these diseases.
Chemokine Receptor Expression on Immune Cells and Anti-Tumor Immunity
Chemokine receptors, present on the surface of immune cells, have a crucial function in controlling immune cell activity and movement within the tumor microenvironment (TME). The expression of chemokine receptors varies among distinct immune cell populations and can be influenced by the TME.
Chemokine receptors are classified into four groups based on their structure and function. Group 1 receptors, CXCR3 and CXCR6, promote the infiltration of T cells and NK cells into the tumor microenvironment. Group 2 receptors, CCR4 and CCR8, expressed on regulatory T cells and Th2 cells, can have an immunosuppressive effect on the anti-tumor immune response. Group 3 receptors, CXCR4 and CCR7, influence the migration and activation of dendritic cells and T cells to the lymph nodes. Group 4 receptors, CX3CR1, participate in the recruitment of monocytes, macrophages, and dendritic cells to the TME. Optimizing these receptors can enhance anti-tumor immune responses.
The expression of chemokine receptors on immune cells can influence their function within the TME. For example, the expression of CXCR3 and CCR5 on T cells has been associated with a favorable prognosis in several types of cancer. In contrast, the upregulation of CCR4 and CCR8 on regulatory T cells has been linked to immune suppression and poor outcomes.
Modulating the expression of chemokine receptors on immune cells represents a potential strategy for enhancing the anti-tumor immune response. For example, targeting CCR4 and CCR8 on regulatory T cells may help reduce their suppressive effects, while upregulating CXCR3 and other receptors on T cells could promote their infiltration into the TME and enhance their activity.
Chemokine receptor-targeted therapies show promise in diverse diseases.
Chemokine receptors play a crucial role in various physiological and pathological processes, including cancer, inflammation, and viral infections. Targeting chemokine receptors with small molecule inhibitors has emerged as a promising therapeutic strategy for these diseases.
In cancer, chemokine receptors play a vital role in tumor progression, metastasis, and immune evasion. Small molecule inhibitors targeting these receptors, such as CXCR4 and CCR5, have shown potential in preclinical and clinical studies to inhibit cancer cell migration and proliferation, enhance immune responses, and sensitize tumors to chemotherapy and immunotherapy.
In inflammation, chemokine receptors regulate immune cell recruitment and activation at the site of inflammation. Small molecule inhibitors targeting chemokine receptors, such as CXCR1 and CXCR2, have demonstrated efficacy in preclinical models of inflammatory diseases, including rheumatoid arthritis and inflammatory bowel disease.
In viral infections, chemokine receptors are essential for the entry and replication of certain viruses, such as HIV. Small molecule inhibitors targeting chemokine receptors, such as CCR5, have been approved for the treatment of HIV/AIDS and are being investigated for other viral infections, including hepatitis C and respiratory viral infections.
Overall, chemokine receptor-targeted therapies hold great promise for treating cancer, inflammation, and viral infections. Further research is needed to optimize their therapeutic potential and fully understand their mechanisms of action and potential side effects.
Small molecule inhibitors targeting chemokine receptors
Chemokine receptors are a class of G protein-coupled receptors (GPCRs) that play a crucial role in the regulation of immune cell migration and trafficking. The dysregulation of chemokine signaling has been associated with various diseases, including cancer, autoimmune disorders, and inflammation. Small molecule inhibitors targeting chemokine receptors have emerged as promising therapeutic agents to treat these diseases. Here are some examples of small molecule inhibitors targeting chemokine receptors:
Maraviroc: It is a small molecule inhibitor that targets CCR5, a chemokine receptor responsible for recruiting immune cells like T cells and macrophages to areas of inflammation. It works by obstructing the interaction between CCR5 and its ligands, which effectively halts immune cell migration. This drug has been approved by the FDA for treating HIV/AIDS as it blocks viral entry into host immune cells. This makes Maraviroc a crucial therapeutic tool for managing and mitigating the progression of the disease.
AMD3100: It is a small molecule inhibitor that specifically targets CXCR4, a chemokine receptor that plays a vital role in the migration of immune cells such as lymphocytes and hematopoietic stem cells. By hindering the interaction between CXCR4 and its ligand, AMD3100 effectively impedes immune cell migration. Researchers are investigating this drug’s potential to treat cancer, as it may prevent cancer cell metastasis by blocking CXCR4 signaling. Therefore, AMD3100 holds promise as a therapeutic agent for combating cancer and other diseases associated with chemokine receptor dysregulation.
TAK-779: It is a small molecule inhibitor that targets both CCR5 and CCR2, two chemokine receptors involved in immune cell recruitment to sites of inflammation. Specifically, CCR2 is responsible for monocyte migration. TAK-779 effectively inhibits the interaction between these receptors and their respective ligands, preventing monocyte migration. Researchers are studying its potential to treat autoimmune diseases since monocytes play a crucial role in these conditions. Thus, TAK-779 may hold promise as a therapeutic agent for managing autoimmune disorders.
Reparixin: It is a small molecule inhibitor that selectively targets CXCR1 and CXCR2, two chemokine receptors responsible for neutrophil recruitment to sites of inflammation. By inhibiting the interaction between these receptors and their ligands, Reparixin effectively prevents neutrophil migration. Researchers are exploring its potential to treat cancer and inflammation, as neutrophil infiltration plays a critical role in these diseases. Therefore, Reparixin holds promise as a therapeutic agent for managing cancer and inflammation associated with chemokine receptor dysregulation.
In summary, small molecule inhibitors targeting chemokine receptors hold great promise for treating a range of diseases by inhibiting immune cell migration to sites of inflammation. Nonetheless, further research is needed to fully comprehend their therapeutic potential and potential side effects.
Chemokine Receptors as Therapeutic Targets in Breast Cancer
Given the critical roles of chemokines and their receptors in breast cancer progression and metastasis, targeting these molecules has emerged as a promising strategy for breast cancer therapy. Several chemokine receptors, such as CXCR4, CCR2, and CCR5, have been identified as potential targets for breast cancer therapy.
CXCR4 is a particularly attractive target for breast cancer therapy, as it is frequently overexpressed in breast cancer cells and is associated with poor prognosis. CXCR4 antagonists, such as AMD3100 and CTCE-9908, have been shown to inhibit breast cancer cell migration, invasion, and metastasis in preclinical studies. Clinical trials evaluating CXCR4 antagonists in breast cancer are currently ongoing.
Similarly, CCR2 and CCR5 antagonists have shown promise in preclinical studies as potential breast cancer therapies. For example, the CCR2 antagonist CCX872 has been shown to inhibit breast cancer cell migration and invasion, as well as tumor-associated macrophage recruitment, in preclinical studies. Clinical trials evaluating CCR2 and CCR5 antagonists in breast cancer are also ongoing.
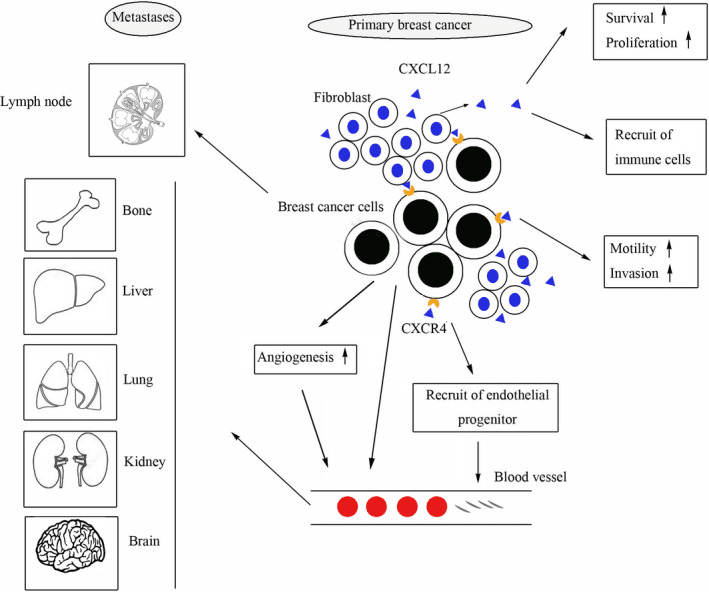
Figure 2. The role of CXCL12/ CXCR4 in breast cancer [3]
The Role of Chemokines in Breast Cancer Progression and Metastasis
Breast cancer cell proliferation, invasion, and metastasis can be facilitated by chemokines and their receptors. Overexpression of CXCL12 (also called stromal-derived factor-1 or SDF-1) and its receptor CXCR4 in breast cancer cells is commonly linked to unfavorable prognosis. Research indicates that CXCL12/CXCR4 signaling plays a crucial role in promoting breast cancer cell migration, invasion, and metastasis to remote areas, including bone, lung, and liver.
Likewise, breast cancer progression and metastasis have been associated with the chemokine CCL2 (also called monocyte chemoattractant protein-1 or MCP-1) and its receptor CCR2. Research suggests that CCL2/CCR2 signaling can facilitate breast cancer cell proliferation, migration, and invasion, in addition to promoting angiogenesis and recruiting tumor-associated macrophages.
Chemokines and their corresponding receptors, namely CXCL1/CXCR2, CXCL8/CXCR1/2, and CCL5/CCR5, have been linked to the development and progression of breast cancer. These signaling molecules and receptors have demonstrated the ability to boost breast cancer cell survival, migration, proliferation, invasion, and angiogenesis, while also promoting immune evasion and the recruitment of tumor-associated macrophages.
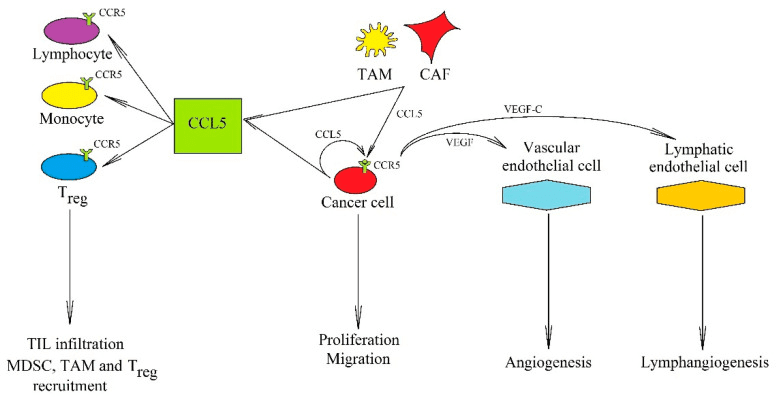
Figure 3. The significance of the CCL5→CCR5 axis in cancer processes [8]
Based Therapies for Breast Cancer Treatment
In addition to chemokine receptor antagonists, several other chemokine-based therapies have been developed for breast cancer treatment. These include chemokine-based vaccines, chemokine-based chimeric antigen receptor (CAR) T-cell therapy, and chemokine-based nanoparticle drug delivery.
Chemokine-based vaccines aim to stimulate the immune system to recognize and attack cancer cells by targeting specific chemokine receptors expressed on tumor cells. For example, a vaccine targeting the chemokine receptor CCR4 has been developed for the treatment of breast cancer. Preclinical studies have shown that this vaccine can induce potent anti-tumor immune responses and inhibit breast cancer growth and metastasis in animal models.
Similarly, chemokine-based CAR T cell therapy involves genetically modifying T cells to express CARs that target specific chemokine receptors expressed on tumor cells. For example, CAR T cells targeting CXCR4 and CCR2 have been developed for the treatment of breast cancer. Preclinical studies have shown that these CAR T cells can selectively target and kill breast cancer cells expressing CXCR4 or CCR2 and inhibit breast cancer growth and metastasis in animal models.
Chemokine-based nanoparticle drug delivery involves encapsulating chemokine receptor antagonists or other anti-cancer drugs in nanoparticles that can selectively target and deliver drugs to tumor cells expressing specific chemokine receptors. For example, CXCR4-targeted nanoparticles loaded with paclitaxel have been developed for the treatment of breast cancer. Preclinical studies have shown that these nanoparticles can selectively target and deliver paclitaxel to breast cancer cells expressing CXCR4 and inhibit breast cancer growth and metastasis in animal models.
Challenges and Opportunities in Developing Chemokine-Based Breast Cancer Therapies
Despite the promising results of preclinical studies, several challenges remain in the development of chemokine-based breast cancer therapies. One major challenge is the potential for off-target effects, as chemokines and chemokine receptors are also involved in normal physiological processes. Therefore, the selective targeting of cancer cells expressing specific chemokine receptors is critical for minimizing off-target effects and maximizing therapeutic efficacy.
Another challenge is the heterogeneity of breast cancer, as different subtypes of breast cancer may express different chemokines and chemokine receptors. Therefore, the development of personalized chemokine-based therapies that target specific chemokines and chemokine receptors expressed in individual breast cancer patients may be necessary for maximizing therapeutic efficacy.
Finally, the optimal combination of chemokine-based therapies with other conventional therapies, such as chemotherapy, radiation therapy, and immunotherapy, remains to be determined. The combination of chemokine-based therapies with conventional therapies may enhance therapeutic efficacy and overcome treatment resistance.
In summary, chemokines and chemokine receptors play critical roles in breast cancer progression and metastasis and offer promising targets for the development of new therapies for this disease. The development of personalized chemokine-based therapies that account for the heterogeneity of breast cancer and the unique chemokine receptor expression profiles of individual patients may be necessary for maximizing therapeutic efficacy. The combination of chemokine-based therapies with other conventional therapies may offer a synergistic approach to breast cancer treatment. With continued research and development, chemokine-based therapies may offer a new strategy for the treatment of breast cancer, ultimately improving patient outcomes and quality of life.
Combining Chemokine-Based Immunotherapies with Conventional Cancer Treatments
To boost the effectiveness of cancer therapy, combining chemokine-based immunotherapies with traditional treatments is a viable strategy. Chemotherapy and radiation therapy can stimulate immune cell infiltration and activation in the tumor microenvironment by releasing tumor-associated antigens and inflammatory signals. Chemokine-based therapies can improve this response and encourage tumor clearance.
For example, combining chemotherapy with CCL21 gene therapy has been found to improve T-cell infiltration and antitumor activity in preclinical melanoma and breast cancer models. Similarly, the use of a CXCR4 antagonist with radiation therapy has demonstrated improved T-cell infiltration and response to immunotherapy in preclinical lung cancer models.
Combining chemokine-based immunotherapies with immune checkpoint inhibitors represents another potential strategy for enhancing the anti-tumor immune response. Chemokine agonists or antagonists can be used to modulate the chemokine signaling pathway and promote the infiltration and activation of immune cells within the TME, while immune checkpoint inhibitors can block inhibitory signals that suppress immune cell activity.
Overall, combining chemokine-based immunotherapies with conventional cancer treatments shows promise as an effective strategy for improving the efficacy of cancer therapy.
Future directions in the development of chemokine receptor-targeted therapies
The field of chemokine receptor-targeted therapies is rapidly evolving, with promising future directions to enhance the efficacy and safety of these treatments.
One direction is the development of more selective inhibitors that only target specific receptors without affecting others. Advances in drug discovery and computational modeling techniques are helping to identify more selective compounds with improved pharmacokinetic and pharmacodynamic properties.
Another direction is the development of combination therapies that target multiple receptors or signaling pathways. Combining chemokine receptor inhibitors with other targeted therapies or immunotherapies may have synergistic effects and improve treatment outcomes.
Furthermore, there is growing interested in the development of cell-based therapies that target chemokine receptors. For example, chimeric antigen receptor (CAR) T cells can be engineered to express receptors that specifically target tumor-associated chemokine receptors, and preclinical studies have shown promising results in cancer treatment.
Finally, there is a need for more robust animal models that accurately mimic human diseases. Advances in genetically engineered animal models and humanized mouse models may provide more accurate predictions of the efficacy and safety of chemokine receptor-targeted therapies in humans.
To conclude, the field of chemokine receptor-targeted therapies is constantly evolving, and future directions involve the creation of more specific inhibitors, combination therapies, cell-based treatments, and enhanced animal models. These advancements offer the potential to increase the effectiveness and safety of these therapies, expanding their applications to a broader range of diseases.
Conclusion
In conclusion, the tumor microenvironment plays a critical role in tumor progression, invasion, and metastasis. Chemokines are key signaling molecules that regulate immune cell recruitment to the TME. Dysregulation of chemokine receptor signaling is implicated in various pathological conditions, including cancer. Chemokine receptor-targeted therapies have emerged as promising approaches for various diseases, including cancer, autoimmune, and inflammatory diseases.
Small molecule inhibitors targeting chemokine receptors have shown therapeutic efficacy in preclinical and clinical studies, and chemokine receptors such as CXCR4 and CCR7 are overexpressed in breast cancer, making them attractive targets for breast cancer therapy. However, the development of chemokine-based therapies for breast cancer faces challenges, such as identifying the appropriate target chemokine receptor and understanding the complex signaling pathways involved in tumor progression and metastasis.
Future directions in developing chemokine receptor-targeted therapies for cancer treatment involve identifying new chemokine receptors and developing more accurate predictive biomarkers. Understanding the intricate relationship between the tumor microenvironment and chemokine receptor signaling is critical in improving cancer treatment outcomes. Personalized chemokine-based therapies based on individual patient characteristics can provide customized treatments, leading to better outcomes. Further research is necessary to optimize combination therapies, overcome immunotherapy resistance, and determine the most effective timing and dosing of chemokine receptor-targeted therapies. The development of innovative delivery methods and strategies for improving drug efficacy and reducing toxicity is also essential in advancing the field of chemokine receptor-targeted therapies. With these advancements, chemokine receptor-targeted therapies may provide new opportunities for improving cancer treatment outcomes.
Combining chemokine-based immunotherapies with conventional cancer treatments like chemotherapy and radiotherapy has the potential to improve cancer treatment efficacy. However, overcoming immunotherapy resistance and optimizing combination therapies require further research. Targeting chemokine receptors presents promising opportunities in cancer therapy, and its potential impact on improving patient outcomes cannot be understated. The development of novel chemokine receptor-targeted therapies and the identification of new predictive biomarkers will contribute significantly to the optimization of combination therapies. With the continued advancement of research and technology, chemokine receptor-targeted therapies offer hope in providing better outcomes for cancer patients.