Repurposing Fludarabine: A Promising Multi-Targeted Approach Against Monkeypox Virus
Abstract
This article explores the potential of Fludarabine as a multi-targeted antiviral agent against the monkeypox virus, leveraging computational drug repurposing. Given the limited treatment options for monkeypox, this study focuses on inhibiting key viral proteins—A6R, D8L, and F13L—which are essential for viral replication, entry, and maturation. Through molecular docking, molecular dynamics (MD) simulation, and MM/GBSA binding affinity analyses, Fludarabine demonstrated a strong binding affinity and stability with the A6R protein, showing potential to disrupt viral replication. Additionally, the compound displayed promising interactions with D8L and F13L, suggesting it may interfere with virus entry and maturation. These findings support further in vitro and in vivo studies on Fludarabine’s efficacy as a monkeypox treatment. This computational approach highlights the value of repurposing existing drugs to respond swiftly to emerging infectious diseases.
Introduction to Monkeypox and the Search for Treatments
Monkeypox, a zoonotic disease, has recently attracted global attention due to a notable resurgence in non-endemic regions. Originating in Central and West Africa, monkeypox belongs to the Orthopoxvirus genus, which includes smallpox (variola) and cowpox viruses. Initially discovered in 1958 in laboratory monkeys and first observed in humans in the early 1970s, the virus typically spreads through direct contact with infected animals or individuals. However, recent outbreaks have highlighted the potential for broader transmission patterns, sparking significant concerns regarding global health.
The virus can cause fever, lymphadenopathy, and distinctive skin lesions, which can progress through various stages. While most cases resolve on their own, some individuals, particularly those with compromised immune systems, may experience severe symptoms or complications. Currently, there is no treatment specifically approved to target monkeypox infection, leaving supportive care as the primary means to manage symptoms. Traditional antiviral therapies, such as Cidofovir and Tecovirimat, have shown effectiveness against poxviruses in laboratory settings, but these treatments are often limited in accessibility and can have significant side effects, making them unsuitable for widespread use against monkeypox.
Given the rapid spread and impact of monkeypox, there is an urgent need to identify effective, safe treatments to mitigate transmission and reduce symptoms. Researchers are now turning to computational methods for rapid drug discovery, particularly targeting key viral proteins essential for monkeypox replication and cell infection. These approaches can rapidly screen large libraries of existing drugs, identifying candidates with strong binding affinities to viral proteins, thus accelerating the development of potential therapies. This strategy is particularly valuable in identifying repurposed drugs—approved medications for other diseases that could effectively treat monkeypox as well. Fludarabine, an anticancer drug, is one such promising candidate that computational methods have highlighted as a potential inhibitor of key monkeypox virus proteins. If proven effective, such drugs could provide a critical tool in managing and controlling monkeypox outbreaks.
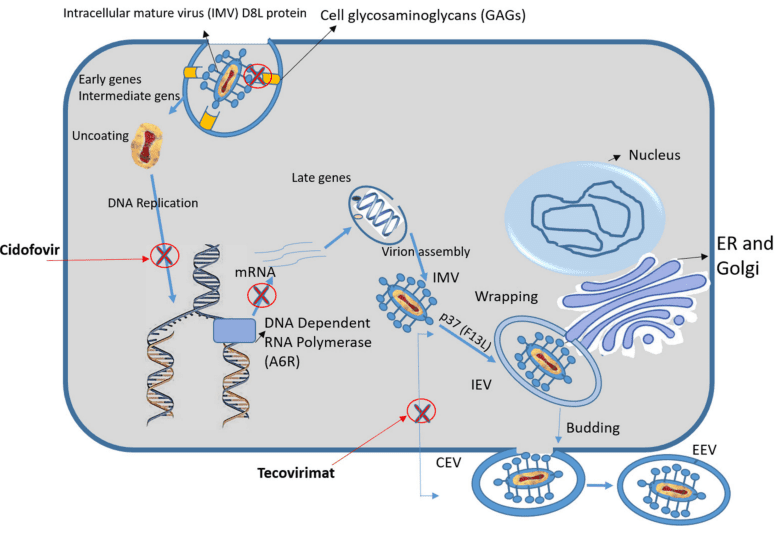
Figure 1. Schematic representation of the MPXV replication cycle, showing the protein targets of antiviral agents (Cidofovir and Tecovirimat) and the studied compound (Fludarabine); the antiviral targets are indicated by red crosses.
Research Focus: Targeting Monkeypox Viral Proteins
The search for effective treatments against monkeypox has led researchers to focus on essential viral proteins that play critical roles in the virus’s replication and infection processes. These proteins offer specific binding sites that, if inhibited, can significantly hinder the virus’s life cycle, reducing its ability to spread and infect host cells. The current research identifies three key proteins within the monkeypox virus: A6R, D8L, and F13L, all of which are fundamental to its survival and replication in human hosts.
The A6R protein, a DNA-dependent RNA polymerase, is crucial for the virus’s replication. This enzyme facilitates the transcription of viral genes, an essential step in viral proliferation. Inhibiting A6R can effectively block the virus’s replication machinery, stopping it from producing necessary components for creating new virions. The second protein, D8L, is involved in the virus’s entry into host cells. D8L binds to cell surface receptors, enabling the virus to attach and initiate infection. Targeting D8L could prevent the virus from penetrating host cells, which is a promising approach for halting early-stage infections.
Finally, the F13L protein is integral to the virus’s maturation and envelopment, enabling the formation of intracellular mature virions (IMVs). These virions can either remain within the host cell or be released to infect neighboring cells, making F13L a valuable target for preventing virus dissemination. The protein plays a vital role in catalyzing the wrapping of virions with membranes, a necessary step for the formation of infectious particles. Fludarabine, an FDA-approved anticancer agent, has shown promising potential in inhibiting these proteins based on computational modeling and molecular docking studies, suggesting that it could effectively disrupt multiple stages of the monkeypox virus’s lifecycle. By focusing on these three proteins, researchers can target fundamental aspects of the monkeypox virus’s replication and infection processes. Such targeted inhibition has the potential to reduce viral load, limit transmission, and contribute to more effective therapeutic strategies against monkeypox.
Discovery and Computational Screening of Fludarabine
To accelerate the identification of potential treatments for monkeypox, researchers have leveraged computational drug discovery methods. These approaches enable the rapid screening of large libraries of FDA-approved drugs to assess their efficacy against specific viral proteins. In this study, researchers used a virtual screening process to evaluate 1,615 FDA-approved compounds against monkeypox proteins, focusing particularly on the DNA-dependent RNA polymerase (A6R), which plays a vital role in viral replication. Among the screened compounds, Fludarabine, an anticancer drug, emerged as a promising candidate due to its high binding affinity for the A6R protein. This discovery points to Fludarabine’s potential to disrupt monkeypox virus replication by inhibiting essential viral functions.
Fludarabine is known for its efficacy in treating chronic lymphocytic leukemia (CLL), where it works as a nucleotide analog, mimicking natural nucleotides to inhibit DNA synthesis in cancer cells. The drug’s mechanism involves converting to its active form, Fludarabine triphosphate, which then disrupts DNA replication. By repurposing Fludarabine for antiviral applications, this study highlights how nucleotide analogs might also interfere with viral DNA synthesis, a pathway essential for monkeypox virus replication. The computational analyses showed that Fludarabine binds effectively to the A6R protein, achieving a docking score of −7.53 kcal/mol, which is one of the strongest binding affinities observed in the study.
Beyond its activity against A6R, Fludarabine demonstrated promising in-silico interactions with two other viral proteins, D8L and F13L, which are involved in host cell entry and viral maturation. The computational results suggest that Fludarabine may inhibit the virus at multiple stages of its life cycle, further underscoring its therapeutic potential against monkeypox. As a repurposed drug, Fludarabine’s safety profile and pharmacokinetics are well-documented, making it a practical candidate for rapid clinical evaluation as a potential monkeypox treatment. The study’s findings recommend further in vitro and in vivo studies to validate Fludarabine’s antiviral effects against monkeypox.
Validation Through Molecular Dynamics and Binding Affinity Analyses
To assess the stability and effectiveness of Fludarabine in binding to key monkeypox proteins, researchers employed molecular dynamics (MD) simulations and MM/GBSA (Molecular Mechanics Generalized Born Surface Area) binding affinity analyses. These computational methods help validate docking results by simulating the interactions between the drug and the viral proteins over time. In this study, Fludarabine’s complex with the DNA-dependent RNA polymerase (A6R) showed exceptional stability throughout the MD simulation, suggesting it could effectively inhibit the protein’s function in a real-world biological environment. The MM/GBSA binding energy of Fludarabine with A6R also demonstrated a strong binding affinity, supporting its potential as a viable treatment option.
Fludarabine also exhibited stable interactions with two other monkeypox proteins, D8L and F13L, although with slightly lower binding energies than A6R. The MD simulation of the Fludarabine-D8L complex revealed consistent hydrogen bonds with essential residues, which are key to inhibiting viral entry into host cells. Meanwhile, the interaction with F13L, a protein involved in virus maturation and envelopment, displayed stable hydrogen bonding, suggesting Fludarabine could interfere with the formation of infectious viral particles.
The MM/GBSA analyses further confirmed Fludarabine’s binding stability across these proteins by estimating the relative binding affinities. The lower free energy values observed in the A6R-Fludarabine complex indicate a stronger, more stable interaction, reinforcing the initial docking results. Moreover, the relatively high binding energy for D8L supports the hypothesis that Fludarabine may block viral entry, while its stable interactions with F13L suggest it could prevent the maturation of viral particles, making it a multi-targeted approach to monkeypox treatment. These findings lay a foundation for further in vitro and in vivo research to validate Fludarabine’s antiviral effects.
Conclusion: Future Steps and Potential of Fludarabine as an Antiviral Agent
The results of this computational study demonstrate the potential of Fludarabine as an antiviral treatment for monkeypox, targeting multiple stages of the virus’s life cycle. Fludarabine’s stable interactions with the A6R, D8L, and F13L proteins suggest it could inhibit monkeypox replication, entry, and maturation processes. This multi-targeted approach is valuable, as targeting various steps in the viral cycle can help limit resistance development and increase treatment efficacy.
The strong binding affinity of Fludarabine with A6R highlights its potential as a potent inhibitor of monkeypox replication. This is particularly significant given the drug’s known safety profile, as it is already approved for use in cancer patients. Repurposing Fludarabine could thus allow for a faster clinical application, especially in outbreak situations where time is critical. Given the robust results obtained in silico, the next step is to validate these findings in vitro, through cell-based assays, and in vivo, using animal models to confirm Fludarabine’s antiviral efficacy against monkeypox.
Additionally, studies exploring Fludarabine’s pharmacokinetics and toxicity in monkeypox models will be essential to assess its suitability for treating viral infections. Such research could pave the way for Fludarabine to become a part of the therapeutic arsenal against monkeypox and potentially other Orthopoxvirus-related disease. With computational drug repurposing approaches proving effective in identifying potential antiviral compounds, Fludarabine represents a promising option in the search for new treatments against emerging infectious diseases.
References
- Altayb, H. N. (2022). Fludarabine, a Potential DNA-Dependent RNA Polymerase Inhibitor, as a Prospective Drug against Monkeypox Virus: A Computational Approach. Pharmaceuticals, 15(1129), 1–18.
- Centers for Disease Control and Prevention. (2022). Treatment | Monkeypox | Poxvirus | CDC.
- Choudhary, M. I., Shaikh, M., Wahab, A., & Rahman, A. (2020). In silico identification of potential inhibitors of key SARS-CoV-2 3CL hydrolase (Mpro) via molecular docking, MMGBSA predictive binding energy calculations, and molecular dynamics simulation. PLoS ONE, 15(6), e0235030.
- Hsiao, J.-C., Chung, C.-S., & Chang, W. (1999). Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. Journal of Virology, 73(10), 8750–8761.
- Reynolds, S. E., & Moss, B. (2015). Characterization of a large, proteolytically processed cowpox virus membrane glycoprotein conserved in most chordopoxviruses. Virology, 483, 209–217.
- Schoeniger, J. S., Hadi, M. Z., Light, Y. K., & Roe, D. C. (2008). Structural basis of poxvirus interaction with cell-surface receptors and synthetic ligands. Sandia National Laboratories.
- Shiryaev, V. A., Skomorohov, M. Y., & Leonova, M. V. (2021). Adamantane derivatives as potential inhibitors of p37 major envelope protein and poxvirus reproduction: Design, synthesis, and antiviral activity. European Journal of Medicinal Chemistry, 221, 113485.
- Gandhi, V., & Plunkett, W. (2002). Cellular and clinical pharmacology of fludarabine. Clinical Pharmacokinetics, 41(2), 93–103.