Resmetirom: A Breakthrough Treatment for MASH and the Future of Liver Disease Management
Abstract
Metabolic dysfunction-associated steatohepatitis (MASH) represents a growing public health concern due to its association with obesity, type 2 diabetes, and metabolic dysfunction. The recent FDA approval of Resmetirom (MGL-3196) marks a significant milestone in MASH treatment. Resmetirom is a thyroid hormone receptor beta (THR-β) agonist that targets the liver to reduce hepatic fat accumulation, enhance lipid metabolism, and improve liver function. Clinical trials have shown promising results, demonstrating the drug’s ability to reduce liver fat, improve liver stiffness, and address key metabolic parameters like cholesterol and triglycerides. This article explores the role of Resmetirom in managing MASH, its clinical trial outcomes, and the potential for combination therapies to optimize treatment. Despite its success, challenges such as long-term efficacy, safety, and the need for non-invasive diagnostic tools remain. Continued research and innovation will be essential in improving patient outcomes and tackling the burden of liver disease globally.
Introduction to Metabolic Dysfunction-Associated Steatohepatitis (MASH)
Metabolic dysfunction-associated steatohepatitis (MASH) is a liver condition that has gained significant attention due to its rising prevalence and association with serious health risks. Formerly recognized as non-alcoholic steatohepatitis (NASH) and non-alcoholic fatty liver disease (NAFLD), the updated terminology reflects a deeper understanding of the disease’s connection to metabolic dysfunction. The shift to using metabolic dysfunction-associated steatotic liver disease (MASLD) and MASH underscores the growing recognition that these conditions are linked to metabolic factors, particularly obesity, type 2 diabetes, and insulin resistance.
Globally, it is estimated that approximately one-third of the population suffers from fatty liver disease, with a significant proportion progressing to steatohepatitis. MASH is a progressive form of liver disease that involves fat accumulation in the liver, inflammation, and damage to liver cells. If left untreated, MASH can lead to severe complications such as cirrhosis, liver failure, and even liver cancer. It is increasingly recognized as an important public health issue, especially in individuals with metabolic disorders.
The clinical impact of MASH extends beyond the liver. Studies show that individuals with MASH often experience concurrent conditions such as cardiovascular disease and chronic kidney disease. These comorbidities contribute to a complex health burden, highlighting the need for more precise diagnostic tools and effective treatments. While lifestyle changes, including weight loss and management of diabetes and hypertension, are cornerstones of therapy, pharmacological interventions have become a critical aspect of managing advanced stages of MASH.
The shift in terminology from NAFLD/NASH to MASLD/MASH reflects a better understanding of the underlying pathophysiology. It also emphasizes the need for more targeted treatments. As the prevalence of metabolic dysfunction continues to rise, the focus on MASH is more critical than ever in improving patient outcomes and reducing the burden on healthcare systems worldwide.
The Role of Resmetirom in Treating MASH
Resmetirom (MGL-3196) represents a breakthrough in the treatment of metabolic dysfunction-associated steatohepatitis (MASH), marking the first FDA-approved drug aimed specifically at managing this liver condition. Resmetirom is a liver-targeted, thyroid hormone receptor beta (THR-β) agonist, designed to selectively activate the THR-β receptor found predominantly in liver cells. This mechanism of action is crucial because it enables Resmetirom to influence liver metabolism while minimizing effects on other tissues, particularly the heart and bones, which are more affected by the thyroid hormone receptor alpha (THR-α).
The primary benefit of Resmetirom in treating MASH lies in its ability to reduce liver fat accumulation and enhance lipid metabolism, addressing two central issues in the progression of the disease. By activating the THR-β receptor in liver cells, Resmetirom stimulates fatty acid oxidation and mitochondrial biogenesis, processes that help clear excess fat from the liver. Additionally, Resmetirom has been shown to reduce the production of very low-density lipoprotein (VLDL) and enhance the liver’s capacity to manage cholesterol, which can be particularly beneficial for individuals with concurrent metabolic issues such as obesity and type 2 diabetes.
Clinical trials have demonstrated the potential of Resmetirom to improve liver health significantly. In phase 2 and phase 3 trials, Resmetirom has shown promising results in reducing hepatic fat content and improving liver stiffness, which are key indicators of liver damage and fibrosis. Moreover, it has been associated with a marked reduction in lipid parameters such as LDL cholesterol and triglycerides, which are important cardiovascular risk factors in MASH patients. Resmetirom’s efficacy has led to its accelerated FDA approval for individuals with moderate to advanced liver fibrosis (F2-F3), a key step forward in addressing an unmet clinical need.
While Resmetirom shows considerable promise, long-term studies are needed to further evaluate its impact on liver fibrosis regression, overall survival rates, and its safety profile in diverse patient populations.
Clinical Trials and Key Findings
The development and approval of Resmetirom for the treatment of metabolic dysfunction-associated steatohepatitis (MASH) have been significantly shaped by clinical trials, particularly those in phase 2 and phase 3. These trials have provided crucial data on the drug’s safety, efficacy, and potential to improve liver health in patients with MASH, a progressive liver condition linked to metabolic dysfunction.
One of the most notable studies, the MAESTRO-NAFLD1 phase 3 trial, was pivotal in demonstrating Resmetirom’s ability to reduce hepatic fat content and improve liver stiffness, which are key indicators of liver damage and fibrosis. The trial involved 1,143 patients with non-alcoholic fatty liver disease (NAFLD) and MASH, who were treated with either 80 mg or 100 mg doses of Resmetirom for 52 weeks. The results revealed significant improvements in liver fat content and a reduction in liver stiffness, as well as improvements in other metabolic parameters such as LDL cholesterol and triglycerides. Notably, the study also showed that Resmetirom was well-tolerated, with common treatment-emergent side effects like diarrhea and nausea being mild and transient.
Further reinforcing these findings, a secondary analysis of the MAESTRO-NASH trial also indicated that Resmetirom led to significant MASH resolution without worsening fibrosis in patients, with 25.9% of patients on the 80 mg dose and 29.9% of those on the 100 mg dose showing resolution, compared to just 9.7% in the placebo group. Importantly, these trials did not show any significant increase in the incidence of serious adverse events, supporting the safety profile of the drug for long-term use.
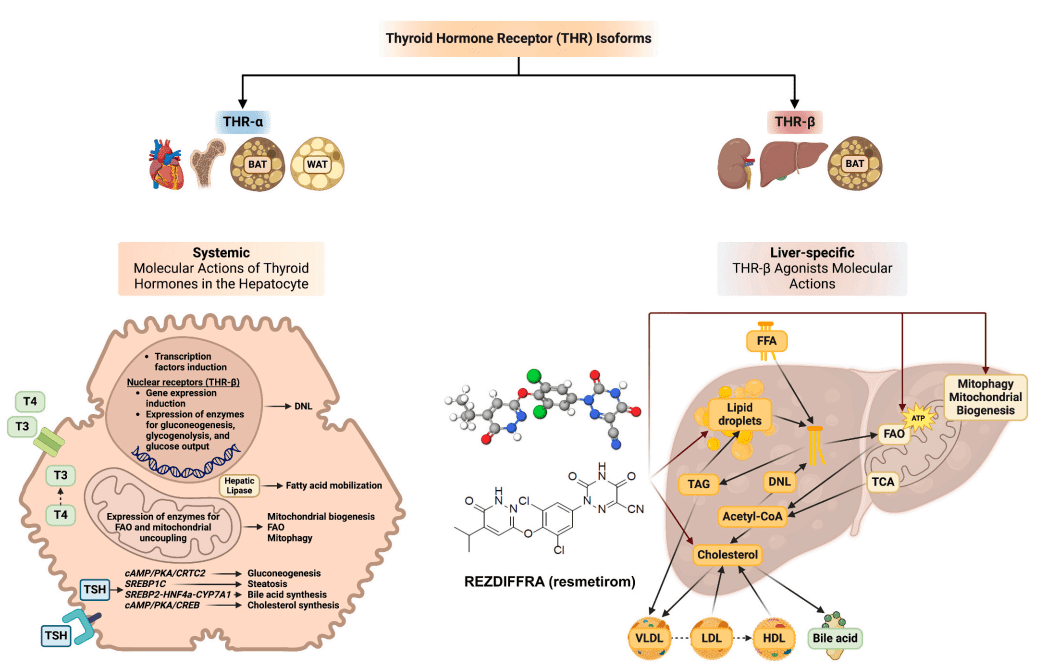
Fig. 1. Molecular actions of thyroid hormones in the hepatocyte and liver-specific molecular actions of resmetirom
As of now, ongoing phase 3 trials, such as MAESTRO-NASH-OUTCOMES, are evaluating Resmetirom’s long-term effects on liver fibrosis and its impact on cardiovascular risk factors, which remain significant in MASH patients. These studies will provide further clarity on the drug’s potential to improve overall patient outcomes and its role in reducing the long-term burden of liver disease.
Future Opportunities and Challenges for MASH Treatment
The approval of Resmetirom as the first FDA-approved treatment for metabolic dysfunction-associated steatohepatitis (MASH) marks a significant step forward in addressing this previously underserved medical need. However, several opportunities and challenges remain in the management of MASH, particularly in refining therapeutic approaches and improving patient outcomes.
One of the key opportunities for the future lies in combination therapies. As Resmetirom targets hepatic fat accumulation and improves lipid metabolism through thyroid hormone receptor beta (THR-β) agonism, combining it with other agents like glucagon-like peptide-1 receptor agonists (GLP-1RAs) could potentially offer synergistic effects. GLP-1RAs, which have shown promise in treating obesity and type 2 diabetes, may complement Resmetirom by addressing metabolic factors that drive liver disease progression, such as insulin resistance and excess body fat. This combination approach could provide a more holistic treatment strategy for MASH patients, potentially reversing liver damage while improving overall metabolic health.
However, challenges persist in fully understanding the long-term impact of Resmetirom and other emerging therapies. Despite encouraging results from clinical trials, further research is required to assess the drug’s effect on liver fibrosis regression and its potential to prevent complications such as cirrhosis and liver cancer. Additionally, the impact of long-term use on cardiovascular risk factors and other health outcomes remains unclear. Moreover, as MASH is closely linked to obesity and type 2 diabetes, addressing these underlying conditions through lifestyle modification and integrated care will be essential to ensure sustained patient benefits.
Lastly, the lack of accurate, non-invasive diagnostic tools for MASH remains a barrier. Many current methods rely on liver biopsy, which can be invasive and pose risks. Efforts to develop more reliable biomarkers and non-invasive imaging techniques will be crucial in identifying at-risk individuals early, optimizing treatment plans, and monitoring response to therapy.
Conclusion: The Path Forward in MASH Management
The approval of Resmetirom has been a landmark moment in the treatment of metabolic dysfunction-associated steatohepatitis (MASH), offering hope for patients suffering from this progressive liver disease. As clinical trials have shown, Resmetirom is effective in reducing hepatic fat content, improving liver stiffness, and addressing key metabolic parameters like cholesterol and triglycerides. While these results are promising, it is clear that further research is necessary to fully understand the long-term benefits and risks of Resmetirom, particularly in relation to liver fibrosis regression and cardiovascular outcomes.
Looking forward, the development of combination therapies and the refinement of diagnostic tools will play crucial roles in enhancing MASH management. The integration of pharmacological treatments with lifestyle interventions will be essential for providing comprehensive care to patients, especially those with concurrent metabolic disorders such as obesity and type 2 diabetes. Additionally, the focus on non-invasive diagnostic methods will help ensure that treatment is initiated early, allowing for better management of the disease and potentially preventing severe complications such as cirrhosis and liver failure.
In conclusion, while significant progress has been made with the approval of Resmetirom, there remains much work to be done in the fight against MASH. Ongoing clinical trials and continued advancements in medical research will be crucial to improving patient outcomes and reducing the long-term burden of liver disease worldwide.
References
Kokkorakis, M., Boutari, C., Hill, M. A., Kotsis, V., Loomba, R., Sanyal, A. J., & Mantzoros, C. S. (2024). Resmetirom, the first approved drug for the management of metabolic dysfunction-associated steatohepatitis: Trials, opportunities, and challenges. Metabolism, 154, 155835. https://doi.org/10.1016/j.metabol.2024.155835
Kokkorakis, M., Boutari, C., Katsiki, N., & Mantzoros, C. S. (2023). From non-alcoholic fatty liver disease (NAFLD) to steatotic liver disease (SLD): An ongoing journey towards refining the terminology for this prevalent metabolic condition and unmet clinical need. Metabolism, 147, 155664. https://doi.org/10.1016/j.metabol.2023.155664
Rinella, M. E., Lazarus, J. V., Ratziu, V., Francque, S. M., Sanyal, A. J., Kanwal, F., et al. (2023). A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Journal of Hepatology, 79, 1542–1556. https://doi.org/10.1016/j.jhep.2023.06.003
Yki-Järvinen, H. (2016). Diagnosis of non-alcoholic fatty liver disease (NAFLD). Diabetologia, 59, 1104–1111. https://doi.org/10.1007/S00125-016-3944-1
Harrison, S. A., Taub, R., Neff, G. W., Lucas, K. J., Labriola, D., Moussa, S. E., et al. (2023). Resmetirom for nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled phase 3 trial. Nature Medicine, 29(12), 2919–2928. https://doi.org/10.1038/S41591-023-02603-1
Hönes, G. S., Sivakumar, R. G., Hoppe, C., König, J., Führer, D., Moeller, L. C., & et al. (2022). Cell-specific transport and thyroid hormone receptor isoform selectivity account for hepatocyte-targeted thyromimetic action of MGL-3196. International Journal of Molecular Sciences, 23(21), 13714. https://doi.org/10.3390/IJMS232213714
Ballinger, S. W., Patterson, C., Knight-Lozano, C. A., Burow, D. L., Conklin, C. A., Hu, Z., et al. (2002). Mitochondrial integrity and function in atherogenesis. Circulation, 106(4), 544–549. https://doi.org/10.1161/01.CIR.0000023921.93743.89