Revolutionizing Breast Cancer Treatment with PARP Inhibitors
Abstract
Breast cancer is a prevalent and challenging disease, necessitating innovative treatments. Traditional therapies like surgery, chemotherapy, and radiation have improved survival but often cause severe side effects, particularly in patients with BRCA1/2 mutations. PARP inhibitors represent a breakthrough, targeting these genetic mutations to induce cancer cell death while sparing healthy cells. Clinical studies have demonstrated the efficacy of PARP inhibitors such as olaparib and talazoparib, improving outcomes and quality of life for patients with BRCA-mutated breast cancer. Despite their promise, resistance development poses a challenge, necessitating ongoing research and combination therapies. The future of PARP inhibitors in breast cancer treatment looks promising, offering personalized and effective options that improve patient outcomes and quality of life.
Introduction
Breast cancer remains one of the most common and challenging cancers affecting women worldwide. With millions diagnosed each year, the need for effective and targeted treatments is more pressing than ever. Traditional therapies such as surgery, chemotherapy, and radiation have significantly improved survival rates. However, they often come with severe side effects and are not always effective, especially in cases involving genetic mutations like BRCA1 and BRCA2.
This is where targeted therapies, such as Poly (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, have begun to make a significant impact. PARP inhibitors represent a revolutionary approach to the treatment of breast cancer, particularly for patients with hereditary tumors associated with BRCA1/2 mutations. These inhibitors work by exploiting the inherent weaknesses in cancer cells’ DNA repair mechanisms, leading to the accumulation of DNA damage and ultimately, cell death. This targeted mechanism not only enhances the effectiveness of the treatment but also reduces the collateral damage to healthy cells, minimizing side effects compared to conventional chemotherapy.
PARP inhibitors have garnered attention not just for their innovative approach but also for their efficacy in clinical settings. Studies have shown that these inhibitors can significantly improve outcomes for patients with germline BRCA1/2-associated breast cancer, offering new hope where traditional treatments may fall short. However, the development of resistance to PARP inhibitors remains a challenge, necessitating ongoing research and the exploration of combination therapies to sustain their effectiveness.
In conclusion, the advent of PARP inhibitors marks a pivotal step forward in breast cancer treatment. By targeting specific genetic vulnerabilities in cancer cells, these therapies promise more personalized and effective treatment options, bringing us closer to the ultimate goal of eradicating breast cancer.
Understanding PARP Inhibitors
PARP inhibitors are a class of drugs designed to target and inhibit the enzyme poly (adenosine diphosphate–ribose) polymerase (PARP). PARP plays a critical role in repairing single-strand DNA breaks through the base excision repair pathway. Inhibition of PARP results in the accumulation of single-strand breaks, which subsequently leads to the formation of double-strand breaks during DNA replication. Cancer cells, particularly those with BRCA1 or BRCA2 mutations, are already compromised in their ability to repair double-strand breaks through homologous recombination repair (HRR). Thus, the inhibition of PARP in these cells leads to synthetic lethality, causing cancer cell death while sparing normal cells.
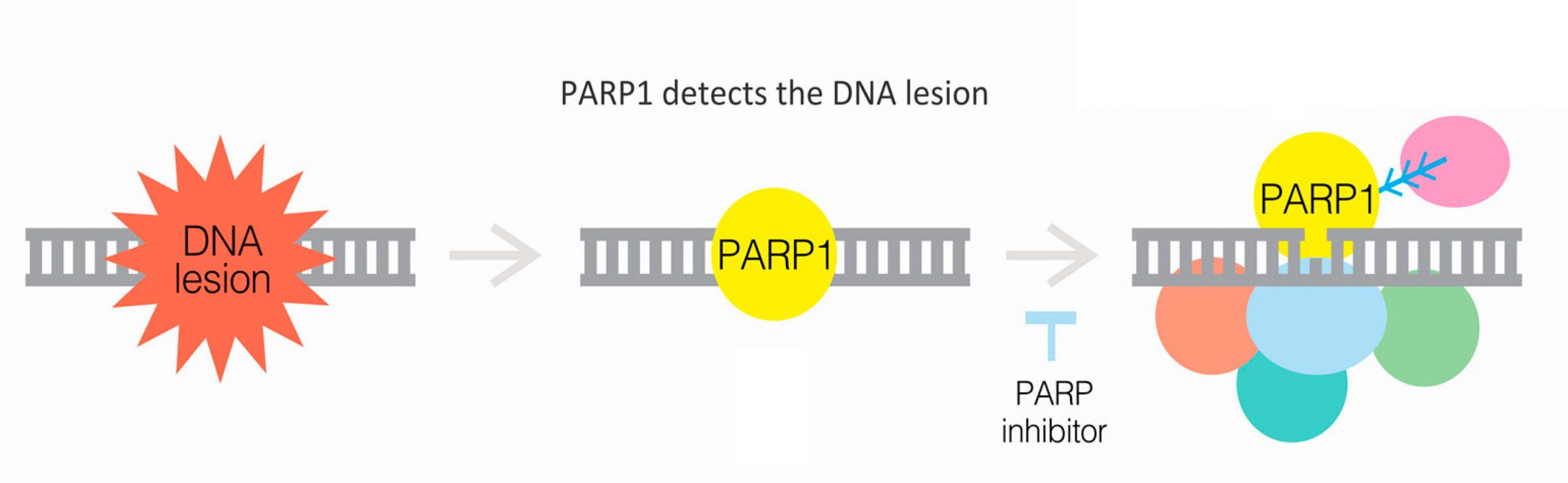
Fig.1 PARP inhibitors work in part by blocking the ability of PARP proteins to repair damaged DNA, which includes recruiting other DNA repair proteins (multicolored circles).
The development of PARP inhibitors is a significant advancement in targeted cancer therapy. Drugs such as olaparib, rucaparib, and talazoparib have been approved for the treatment of breast cancer, particularly in patients with BRCA1/2 mutations. These inhibitors work by specifically targeting the DNA repair weaknesses inherent in cancer cells, leading to their destruction without the widespread damage often caused by conventional chemotherapy. This targeted approach not only improves the efficacy of the treatment but also reduces adverse effects, enhancing the overall quality of life for patients undergoing therapy.
PARP inhibitors are administered orally, offering a convenient and less invasive option compared to traditional intravenous chemotherapy. The clinical benefits of PARP inhibitors have been demonstrated in several studies, showing improved progression-free survival rates in patients with BRCA-mutated breast cancer. These promising results have positioned PARP inhibitors as a critical component of personalized cancer therapy, tailored to exploit specific genetic vulnerabilities in tumors.
The Role of BRCA1/2 Mutations
BRCA1 and BRCA2 are tumor suppressor genes that produce proteins involved in the repair of double-strand DNA breaks through homologous recombination. Mutations in these genes significantly impair DNA repair processes, leading to genomic instability and increased cancer risk. Individuals with germline BRCA1/2 mutations have a higher predisposition to developing breast and ovarian cancers. These mutations are inherited in an autosomal dominant pattern, meaning that a single copy of the mutated gene can increase cancer risk.
The connection between BRCA mutations and breast cancer has profound implications for treatment. Patients with BRCA1/2 mutations often develop more aggressive forms of cancer that are resistant to traditional therapies. The advent of PARP inhibitors has provided a new targeted treatment option specifically for these high-risk patients. By inhibiting PARP, these drugs exploit the existing DNA repair deficiency in BRCA-mutated cancer cells, leading to their selective death.
Understanding the role of BRCA mutations in breast cancer has also highlighted the importance of genetic testing. Identifying individuals with BRCA1/2 mutations allows for personalized treatment plans, including the use of PARP inhibitors, and can inform preventive measures such as increased surveillance or prophylactic surgeries. Genetic counseling and testing have become integral components of managing hereditary breast cancer risk, enabling more effective and tailored interventions.
Efficacy of PARP Inhibitors in Breast Cancer
The introduction of Poly (adenosine diphosphate–ribose) polymerase (PARP) inhibitors has marked a significant advancement in the treatment of breast cancer, particularly for patients with BRCA1 and BRCA2 mutations. These inhibitors have demonstrated remarkable efficacy in both preclinical and clinical settings, offering new hope for improved patient outcomes.
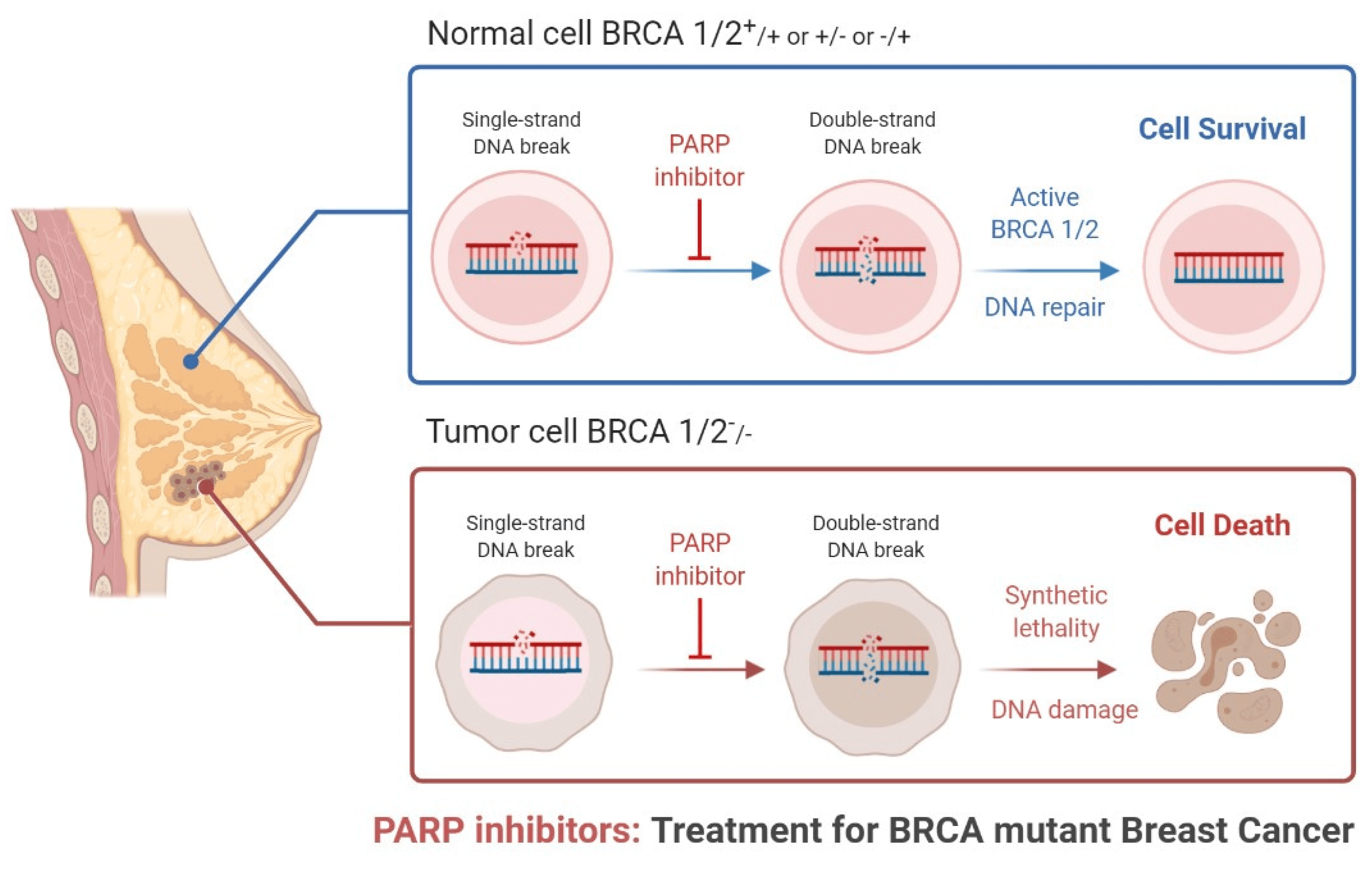
Fig.2 Role of PARP inhibitors in treatments for BRCA mutant breast cancer.
Clinical Trials and Studies
Several landmark clinical trials have established the efficacy of PARP inhibitors in breast cancer treatment. The OlympiAD trial was one of the first to show the benefits of olaparib, a PARP inhibitor, in patients with metastatic breast cancer carrying germline BRCA mutations. The trial demonstrated that patients treated with olaparib had significantly longer progression-free survival compared to those receiving standard chemotherapy.
Another pivotal study, the EMBRACE trial, evaluated talazoparib, another PARP inhibitor, in patients with advanced breast cancer and germline BRCA mutations. Results showed that talazoparib significantly improved progression-free survival and was associated with a better quality of life compared to standard treatments.
Patient Outcomes and Statistics
The effectiveness of PARP inhibitors is particularly pronounced in patients with BRCA-mutated breast cancer, a group that often faces more aggressive disease and poorer prognosis with conventional therapies. By targeting the DNA repair deficiencies in these cancer cells, PARP inhibitors induce synthetic lethality, leading to selective cancer cell death while sparing normal cells. This targeted mechanism not only improves the efficacy of the treatment but also reduces the adverse effects typically associated with chemotherapy.
Clinical data support the use of PARP inhibitors as a standard treatment option for BRCA-mutated breast cancer. For instance, the median progression-free survival for patients treated with olaparib in the OlympiAD trial was 7.0 months compared to 4.2 months for those receiving chemotherapy Similarly, in the EMBRACE trial, the median progression-free survival for patients on talazoparib was 8.6 months compared to 5.6 months with standard therapy.
Expanding Indications
While the primary indication for PARP inhibitors has been for BRCA-mutated breast cancer, research is ongoing to expand their use to other patient populations. For example, studies are exploring the efficacy of PARP inhibitors in patients with homologous recombination deficiency (HRD) beyond BRCA mutations, including those with mutations in other DNA repair genes such as PALB2 and RAD51.
Combination therapies are also being investigated to enhance the efficacy of PARP inhibitors. Combining PARP inhibitors with other agents such as immune checkpoint inhibitors, angiogenesis inhibitors, or DNA-damaging agents like platinum-based chemotherapy could provide synergistic effects, potentially overcoming resistance and improving outcomes.
Future Directions
The future of PARP inhibitor therapy in breast cancer looks promising, with ongoing research aimed at overcoming resistance and expanding their use to a broader patient population. Biomarker-driven approaches are being developed to better identify patients who will benefit from these therapies and next-generation PARP inhibitors are being designed to address resistance mechanisms.
In conclusion, PARP inhibitors have significantly improved the treatment landscape for BRCA-mutated breast cancer. Their ability to target specific genetic vulnerabilities in cancer cells has translated into better clinical outcomes and a new standard of care for this high-risk population. Continued research and innovation hold the promise of further enhancing their efficacy and expanding their benefits to more patients.
Future Directions and Research
The future of PARP inhibitor therapy in breast cancer holds significant promise, with ongoing research aimed at enhancing their efficacy and expanding their use to a broader patient population. One key area of focus is understanding and overcoming resistance mechanisms. As cancer cells develop resistance to PARP inhibitors, researchers are exploring combination therapies that can counteract these adaptations. For example, combining PARP inhibitors with other DNA-damaging agents, such as platinum-based chemotherapies, or with immune checkpoint inhibitors like anti-PD-1 or anti-PD-L1 antibodies, has shown potential in preclinical studies and is currently being tested in clinical trials.
Another promising direction involves targeting the DNA damage response (DDR) pathways. Inhibitors of proteins such as ATR, CHK1, and WEE1 are being evaluated in combination with PARP inhibitors to induce greater DNA damage and impair cancer cell survival more effectively. This approach aims to exploit multiple vulnerabilities in cancer cells, potentially overcoming resistance and improving patient outcomes.
Patient Perspective
For patients, the development and availability of PARP inhibitors represent a significant advancement in personalized medicine. These therapies offer a targeted approach that specifically addresses the genetic vulnerabilities of their tumors, leading to more effective treatment with fewer side effects compared to conventional chemotherapy. Real-life stories and testimonials from patients who have benefited from PARP inhibitors highlight their impact on quality of life and survival rates. Many patients report experiencing fewer adverse effects and a better overall treatment experience.
Moreover, the role of genetic testing in identifying candidates for PARP inhibitor therapy cannot be overstated. By pinpointing individuals with BRCA1/2 mutations or other homologous recombination deficiencies, healthcare providers can tailor treatment plans more effectively, ensuring that patients receive the most appropriate and beneficial therapies. Genetic counseling and testing have thus become integral components of managing hereditary breast cancer risk, enabling personalized treatment and preventive strategies.
Conclusion
The introduction of PARP inhibitors has marked a transformative step in the treatment of breast cancer, particularly for patients with BRCA1 and BRCA2 mutations. These innovative drugs exploit the unique vulnerabilities in cancer cells’ DNA repair mechanisms, leading to targeted cell death while minimizing damage to healthy cells. Clinical trials have consistently demonstrated the efficacy of PARP inhibitors, such as olaparib and talazoparib, in improving progression-free survival and enhancing the quality of life for patients with BRCA-mutated breast cancer.
Despite these advancements, challenges remain, particularly concerning developing resistance to PARP inhibitors. Cancer cells often adapt through various mechanisms, including restoring homologous recombination repair or upregulating drug efflux pumps, which diminish the effectiveness of these therapies. Addressing these resistance mechanisms is a critical focus of ongoing research. Combination therapies, involving PARP inhibitors and other agents like immune checkpoint inhibitors or DNA-damaging drugs, are being actively explored to overcome resistance and achieve more durable responses.
The future of PARP inhibitor therapy looks promising, with efforts directed toward expanding their use beyond BRCA-mutated cancers to other types of homologous recombination deficiencies. Additionally, developing next-generation PARP inhibitors and identifying reliable biomarkers for predicting response and monitoring resistance are key areas of investigation.
For patients, the advent of PARP inhibitors offers a beacon of hope, providing a more personalized and effective treatment option. The role of genetic testing in identifying suitable candidates for PARP inhibitor therapy underscores the importance of precision medicine in cancer care. By tailoring treatments to the genetic profiles of tumors, healthcare providers can significantly improve outcomes and offer more targeted interventions.
In conclusion, while challenges remain, the progress in PARP inhibitor therapy represents a significant leap forward in the fight against breast cancer. Continued research and innovation are essential to fully realize the potential of these therapies and extend their benefits to a broader patient population.
References
- Morganti, S., Marra, A., De Angelis, C., & Toss, A. (2024). PARP Inhibitors for Breast Cancer Treatment: A Review. JAMA Oncology.
- Robson, M., Im, S. A., Senkus, E., Xu, B., Domchek, S. M., Masuda, N., … & Li, W. (2017). Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. New England Journal of Medicine, 377(6), 523-533.
- Litton, J. K., Rugo, H. S., Ettl, J., Hurvitz, S. A., Goncalves, A., Lee, K. H., … & Martin, M. (2018). Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. New England Journal of Medicine, 379(8), 753-763.
- Telli, M. L., Timms, K. M., Reid, J., Hennessy, B., Mills, G. B., Jensen, K. C., … & Ford, J. M. (2016). Homologous recombination deficiency (HRD) status predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clinical Cancer Research, 22(15), 3764-3773.
- Shen, Y., Rehman, F. L., Feng, Y., Boshuizen, J., Bajrami, I., Elliott, R., … & Ashworth, A. (2019). BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clinical Cancer Research, 19(18), 5003-5015.
- Fong, P. C., Boss, D. S., Yap, T. A., Tutt, A., Wu, P., Mergui-Roelvink, M., … & de Bono, J. S. (2009). Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. New England Journal of Medicine, 361(2), 123-134.
- Lord, C. J., & Ashworth, A. (2016). BRCAness revisited. Nature Reviews Cancer, 16(2), 110-120.
- Noordermeer, S. M., & van Attikum, H. (2019). PARP inhibitor resistance: A tug-of-war in BRCA-mutated cells. Trends in Cell Biology, 29(10), 820-834.
- O’Connor, M. J. (2015). Targeting the DNA damage response in cancer. Molecular Cell, 60(4), 547-560.
- Lord, C. J., & Ashworth, A. (2017). PARP inhibitors: Synthetic lethality in the clinic. Science, 355(6330), 1152-1158.




