What is Rufinamide?
Rufinamide is an anticonvulsant drug primarily prescribed for the treatment of Lennox-Gastaut Syndrome (LGS), a rare and severe form of childhood-onset epilepsy that is often resistant to other antiepileptic drugs (AEDs). LGS is characterized by multiple seizure types, cognitive impairments, and a high degree of treatment resistance, which makes it one of the most challenging forms of epilepsy to manage. Rufinamide is also utilized off-label for managing other types of seizures, including partial seizures, though its main indication remains LGS.
Rufinamide’s effectiveness stems from its modulation of sodium channels in the brain. Unlike other anticonvulsants, Rufinamide works by stabilizing the neuronal membranes and reducing the excitability of neurons. Specifically, it binds to and inhibits the sodium channels, preventing the rapid firing of neurons that can lead to seizures. This mechanism of action is particularly beneficial for patients with refractory epilepsy, where traditional treatments may fail to control the frequency and severity of seizures.
This drug was first approved by the U.S. Food and Drug Administration (FDA) in 2008 for the treatment of LGS, and it has since become a critical part of the therapeutic arsenal for managing this difficult-to-treat condition. It is often prescribed as an adjunct therapy, meaning it is used in combination with other anticonvulsants to enhance seizure control.
Rufinamide is typically administered orally, either in tablet or liquid form, and its dosage is individualized based on the patient’s age, weight, and the severity of the condition. The medication is generally well-tolerated, but like all drugs, it can have side effects. Common side effects include dizziness, somnolence (sleepiness), irritability, and gastrointestinal disturbances such as nausea. In rare cases, more serious side effects like QT interval prolongation (which can affect heart rhythm) may occur, requiring monitoring of the patient’s heart function.
Clinical research has demonstrated that Rufinamide significantly reduces seizure frequency in patients with LGS, making it one of the preferred treatments for this condition. Studies also suggest that Rufinamide is generally well-tolerated, with minimal risk for cognitive impairment, a common concern in children with epilepsy. As the drug continues to be studied, its role in treating other forms of epilepsy and potential off-label uses are actively being explored.
Medical Uses of Rufinamide
Rufinamide is a critical medication in the treatment of epilepsy, primarily used for managing Lennox-Gastaut Syndrome (LGS), a severe and debilitating form of childhood-onset epilepsy. LGS is characterized by multiple seizure types, cognitive decline, and significant resistance to conventional antiepileptic drugs (AEDs). Rufinamide was approved by the U.S. Food and Drug Administration (FDA) in 2008 as an adjunctive therapy for LGS and has since become a cornerstone in the management of this challenging condition.
In patients with LGS, Rufinamide has demonstrated remarkable efficacy in reducing seizure frequency, particularly tonic and atonic seizures, which are highly disruptive and difficult to control. Clinical studies have shown that patients treated with Rufinamide experience a significant improvement in seizure management and overall quality of life compared to those on placebo or other AEDs. Rufinamide is also sometimes prescribed for partial seizures, though this is considered an off-label use.
The drug’s mechanism of action—modulating sodium channel activity—plays a critical role in stabilizing neuronal membranes and preventing excessive electrical discharges in the brain. Its unique properties make it especially effective for drug-resistant epilepsy, where traditional therapies fail to achieve adequate control.
Rufinamide is typically used as an adjunct therapy, meaning it is prescribed alongside other AEDs to optimize seizure management. It is particularly valuable in combination regimens for patients who do not respond to monotherapy. Its ability to reduce seizure severity and frequency, combined with a generally favorable safety profile, makes it an essential option for patients with difficult-to-treat epilepsy.
The broader potential of Rufinamide in other epileptic syndromes continues to be explored, with ongoing research seeking to expand its therapeutic applications.
How does Rufinamide Work in the Brain?
Rufinamide is a sodium channel modulator that plays a key role in the management of epilepsy, particularly for patients with Lennox-Gastaut Syndrome (LGS). Its unique mechanism of action involves stabilizing the neuronal membranes by inhibiting excessive and prolonged sodium channel activity. This modulation reduces neuronal excitability, preventing the hyperactive electrical discharges in the brain that lead to seizures.
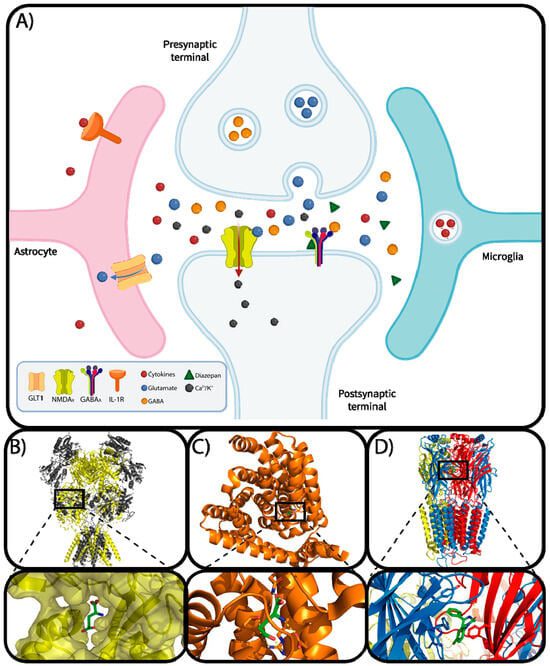
Sodium channels are essential for the generation and propagation of electrical signals in neurons. In individuals with epilepsy, these channels often function abnormally, leading to excessive and unregulated neuronal firing. Rufinamide binds selectively to these sodium channels, particularly in their inactivated state, prolonging their refractory period. By doing so, the drug limits the repetitive firing of neurons and reduces the likelihood of seizures.
This mechanism is particularly effective in tonic and atonic seizures, which are common in LGS and are characterized by sudden muscle stiffening or loss of muscle tone. Rufinamide’s ability to target abnormal neuronal activity without significantly affecting normal brain function makes it a valuable therapy for drug-resistant epilepsy.
In clinical studies, Rufinamide has shown a significant reduction in the frequency and severity of seizures when used as an adjunct therapy. Additionally, its pharmacological profile includes minimal cognitive side effects, making it an appealing option for children and adolescents with epilepsy.
Research continues to explore Rufinamide’s broader applications in treating other epileptic syndromes. As more is understood about its effects on sodium channels and overall neuronal function, Rufinamide may offer additional benefits beyond its current indications.
Conclusion
Rufinamide has emerged as a significant advancement in the management of Lennox-Gastaut Syndrome (LGS), a severe and treatment-resistant form of childhood-onset epilepsy. By effectively reducing the frequency and severity of seizures, particularly tonic and atonic seizures, Rufinamide improves the quality of life for patients and their families. Its unique mechanism of action—modulating sodium channels to prevent excessive neuronal excitability—offers a targeted approach that complements other antiepileptic therapies.
One of the key strengths of Rufinamide is its use as an adjunctive therapy, making it an essential option for patients who do not achieve sufficient seizure control with conventional medications. The drug’s favorable safety profile, including minimal cognitive side effects, ensures that it is suitable for use in children and adolescents, who are disproportionately affected by LGS. Additionally, its oral administration, available in tablet or suspension forms, makes dosing flexible and patient-friendly.
Clinical studies consistently demonstrate Rufinamide’s efficacy in reducing seizure frequency, contributing to its widespread acceptance among healthcare providers. Ongoing research continues to explore its potential applications for other types of epilepsy, providing hope for broader therapeutic benefits in the future.
While Rufinamide is not without potential side effects—such as dizziness, fatigue, and rare cardiac complications—it remains a valuable tool in the arsenal of antiepileptic drugs. For patients with refractory epilepsy, its introduction represents a critical step forward in improving seizure control and overall outcomes.
In conclusion, Rufinamide stands out as a highly effective and well-tolerated treatment for LGS, offering renewed hope for patients and families facing the challenges of epilepsy. Its continued study and integration into personalized treatment plans underscore its importance in the evolving landscape of epilepsy care.




