Ruxolitinib: A Paradigm Shift in Myelofibrosis Treatment
Abstract
Ruxolitinib, an FDA-approved JAK1 and JAK2 inhibitor, has emerged as a cornerstone in the treatment of myelofibrosis. This blog explores the intricate mechanisms by which Ruxolitinib exerts its effects, targeting the JAK-STAT pathway to alleviate disease symptoms and reduce spleen size. Detailed insights into key clinical trials, such as COMFORT-I and COMFORT-II, highlight the significant clinical benefits observed in patients. The discussion extends to ongoing research and the potential future applications of Ruxolitinib, alongside an examination of related chemical compounds including impurities, metabolites, isotope-labeled compounds, and building blocks. This comprehensive overview underscores the critical role of Ruxolitinib in transforming myelofibrosis therapy and the continued importance of research in this field.
Introduction to Ruxolitinib
Ruxolitinib, a potent inhibitor of Janus kinase 1 (JAK1) and Janus kinase 2 (JAK2), represents a major breakthrough in the treatment of myelofibrosis, a type of myeloproliferative neoplasm characterized by the clonal proliferation of hematopoietic stem cells and extensive bone marrow fibrosis. Myelofibrosis manifests clinically with symptoms such as severe anemia, splenomegaly, and debilitating constitutional symptoms, severely affecting patients’ quality of life and overall survival.
Ruxolitinib’s mechanism of action involves the inhibition of the JAK-STAT signaling pathway, which is aberrantly activated in myelofibrosis due to mutations such as JAK2V617F. This pathway is crucial for the regulation of various cytokines and growth factors that contribute to the pathogenesis of the disease. By blocking JAK1 and JAK2, Ruxolitinib effectively reduces the pathological cytokine signaling, leading to a reduction in spleen size and alleviation of systemic symptoms.
The clinical efficacy of Ruxolitinib was demonstrated in pivotal phase III clinical trials, notably COMFORT-I and COMFORT-II. These studies provided robust evidence that Ruxolitinib significantly reduces splenomegaly and improves symptom scores in patients with intermediate-2 and high-risk myelofibrosis. In COMFORT-I, a randomized, double-blind, placebo-controlled trial, patients receiving Ruxolitinib experienced a substantial reduction in spleen volume and a marked improvement in quality of life compared to the placebo group. COMFORT-II, a randomized, open-label trial, compared Ruxolitinib to the best available therapy, confirming the drug’s superior efficacy.
Approved by the U.S. Food and Drug Administration (FDA) in 2011, Ruxolitinib has since become a cornerstone in the management of myelofibrosis, offering a well-tolerated oral therapeutic option for patients. Its approval marked a significant advancement in the treatment paradigm for myelofibrosis, providing a targeted therapy that addresses both the hematologic and symptomatic burdens of the disease. The continued research and development surrounding Ruxolitinib and related JAK inhibitors hold promise for further improvements in the management of myelofibrosis and other related hematologic malignancies.
Mechanism of Action
Ruxolitinib, a selective inhibitor of Janus kinase 1 (JAK1) and Janus kinase 2 (JAK2), exerts its therapeutic effects by targeting the JAK-STAT signaling pathway, which is aberrantly activated in myelofibrosis. The JAK-STAT pathway is critical for mediating the effects of various cytokines and growth factors involved in hematopoiesis, immune function, and inflammation. In myelofibrosis, mutations such as JAK2V617F lead to constitutive activation of this pathway, driving pathological cell proliferation and survival, as well as the production of pro-inflammatory cytokines.

Ruxolitinib binds to the ATP-binding site of JAK1 and JAK2, thereby inhibiting their kinase activity. This inhibition prevents the phosphorylation and activation of signal transducers and activators of transcription (STAT) proteins, particularly STAT3 and STAT5, which are essential for transmitting signals from the cell surface to the nucleus to regulate gene expression. By blocking this signaling cascade, Ruxolitinib reduces the pathological activity of cytokines that contribute to the symptoms and complications of myelofibrosis.
Clinical studies have demonstrated that Ruxolitinib effectively reduces spleen size and improves systemic symptoms such as fatigue, night sweats, and bone pain, which are driven by the elevated cytokine levels in myelofibrosis patients. Additionally, Ruxolitinib has been shown to decrease levels of circulating inflammatory markers, including interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP). These effects underscore the drug’s ability to modulate the inflammatory milieu associated with myelofibrosis.
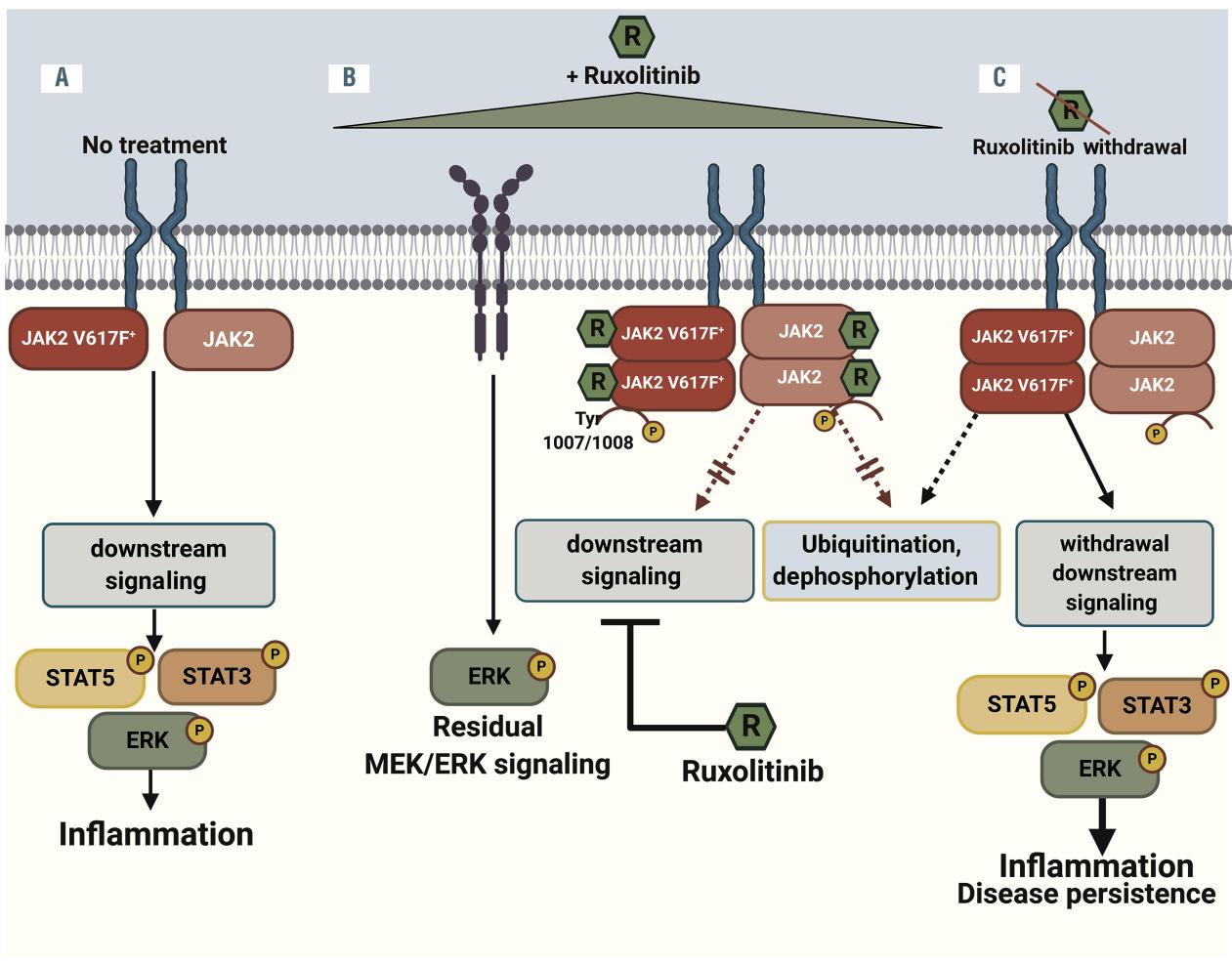
Furthermore, Ruxolitinib’s impact on the JAK-STAT pathway also translates into the attenuation of abnormal hematopoietic stem cell proliferation. This results in clinical benefits such as the reduction of splenomegaly and alleviation of disease-related symptoms. While Ruxolitinib does not cure myelofibrosis or eliminate the malignant clone, its ability to target the underlying pathogenic mechanisms makes it a pivotal therapeutic agent in the management of this chronic and progressive disease.
Clinical Efficacy
Ruxolitinib has demonstrated significant clinical efficacy in the treatment of myelofibrosis, as evidenced by pivotal phase III clinical trials such as COMFORT-I and COMFORT-II. These trials have been instrumental in establishing Ruxolitinib as a transformative therapeutic option for patients with intermediate-2 and high-risk myelofibrosis.
The COMFORT-I trial was a randomized, double-blind, placebo-controlled study that enrolled 309 patients with myelofibrosis. The primary endpoint was the proportion of patients achieving a reduction in spleen volume of 35% or more at 24 weeks, as measured by magnetic resonance imaging (MRI). The results were striking: 41.9% of patients in the Ruxolitinib group achieved this primary endpoint compared to only 0.7% in the placebo group. Additionally, patients treated with Ruxolitinib experienced substantial improvements in myelofibrosis-related symptoms, including fatigue, night sweats, and pruritus, leading to enhanced quality of life.
The COMFORT-II trial was a randomized, open-label study comparing Ruxolitinib with the best available therapy (BAT) in 219 patients. Similar to COMFORT-I, the primary endpoint was the proportion of patients achieving a reduction in spleen volume of 35% or more at 48 weeks. The trial demonstrated that 28.5% of patients in the Ruxolitinib group achieved the primary endpoint, whereas none of the patients in the BAT group did. Furthermore, the trial highlighted the durability of Ruxolitinib’s effects, with sustained spleen volume reduction and symptom improvement observed over time.
Beyond these primary endpoints, both trials reported secondary outcomes that further underscored the benefits of Ruxolitinib. Patients treated with Ruxolitinib exhibited reductions in circulating inflammatory cytokines and improvements in overall survival compared to control groups. Notably, the safety profile of Ruxolitinib was favorable, with manageable adverse effects such as anemia and thrombocytopenia.
These clinical findings have solidified Ruxolitinib’s role in the management of myelofibrosis, providing a much-needed therapeutic option that addresses both the hematologic and symptomatic burdens of the disease. The consistent efficacy and safety outcomes across multiple studies highlight Ruxolitinib’s potential to significantly improve patient outcomes in myelofibrosis.
Research and Development
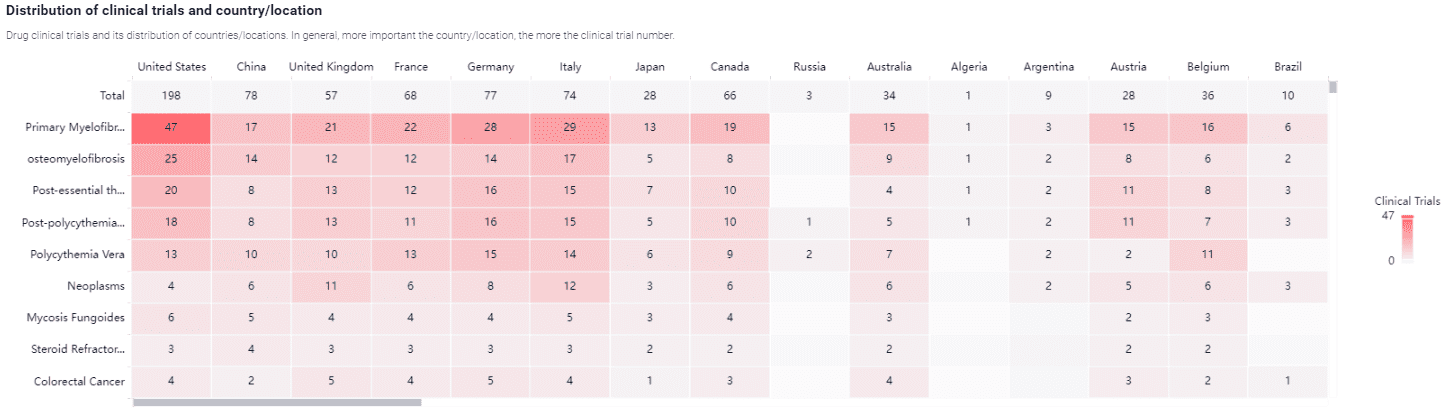
The research and development landscape surrounding Ruxolitinib and related JAK inhibitors continues to evolve, driven by a deeper understanding of the JAK-STAT pathway and its role in myeloproliferative neoplasms. Ruxolitinib’s success in treating myelofibrosis has sparked interest in its potential applications for other hematologic and non-hematologic conditions, as well as the exploration of related compounds such as impurities, metabolites, isotope-labeled compounds, and building blocks.
Ongoing research aims to optimize Ruxolitinib’s therapeutic efficacy and expand its use beyond myelofibrosis. Studies are investigating its application in polycythemia vera and essential thrombocythemia, with promising results. For instance, the RESPONSE trial demonstrated that Ruxolitinib significantly improves hematocrit control and reduces the need for phlebotomy in patients with polycythemia vera. Additionally, there is growing interest in Ruxolitinib’s potential to treat graft-versus-host disease (GVHD) following allogeneic stem cell transplantation, where early-phase trials have shown encouraging outcomes.
The development of novel JAK inhibitors with improved specificity and safety profiles is another active area of research. Agents such as fedratinib and pacritinib are being evaluated in clinical trials, offering alternative treatment options for patients who may not tolerate Ruxolitinib. These newer inhibitors aim to minimize adverse effects while maintaining or enhancing therapeutic benefits.
Isotope-labeled compounds and metabolites of Ruxolitinib are also of significant interest for pharmacokinetic and pharmacodynamic studies. These compounds help elucidate the drug’s metabolic pathways and interactions, providing critical insights into its mechanism of action and potential side effects. Building blocks and related chemical entities are essential for synthesizing these compounds, facilitating advanced research and development efforts.
In parallel, research is focusing on understanding the long-term effects of Ruxolitinib and the mechanisms underlying resistance to therapy. Studies are examining the molecular and genetic factors that contribute to resistance, with the goal of developing combination therapies that can overcome these challenges.
In summary, the research and development efforts surrounding Ruxolitinib are multifaceted, encompassing the exploration of its broader therapeutic applications, the development of next-generation JAK inhibitors, and the study of its metabolites and isotope-labeled compounds. These endeavors aim to enhance our understanding of the drug, improve patient outcomes, and pave the way for new therapeutic strategies in the treatment of myeloproliferative neoplasms and other diseases.
Conclusion
Ruxolitinib has become a cornerstone in treating myelofibrosis, offering significant clinical benefits by targeting the JAK-STAT pathway. Its effectiveness in reducing spleen size and alleviating systemic symptoms has been demonstrated in pivotal clinical trials like COMFORT-I and COMFORT-II. These trials have confirmed Ruxolitinib’s capacity to improve the quality of life for patients with myelofibrosis.
Ongoing research aims to optimize Ruxolitinib’s use and explore additional applications. Newer JAK inhibitors, such as fedratinib and pacritinib, are being developed to provide alternative treatment options. The study of Ruxolitinib’s metabolites and isotope-labeled compounds continues to provide valuable insights into its pharmacokinetics and pharmacodynamics. Future directions include investigating combination therapies to overcome resistance and exploring Ruxolitinib’s use in other myeloproliferative neoplasms and inflammatory conditions. These efforts aim to enhance patient outcomes and expand treatment options.
In summary, Ruxolitinib’s success in managing myelofibrosis marks a significant advancement in hematologic therapy. Continued research and clinical practice integration will maximize its therapeutic potential, improving patient care in this challenging field.