SHP2 and Its Potential Role as a Therapeutic Target in Cancer Treatment
Abstract
SHP2 (also known as PTPN11) is a protein tyrosine phosphatase that is encoded by the PTPN11 gene. It is involved in various signaling pathways, including those mediated by growth factors and cytokines, and plays important roles in cell growth, differentiation, and migration. Mutations in the PTPN11 gene have been linked to several developmental disorders, including Noonan syndrome, LEOPARD syndrome, and some cases of juvenile myelomonocytic leukemia. SHP2 is also a promising target for cancer therapy, as it is frequently activated in many types of tumors and is involved in promoting tumor growth and survival.
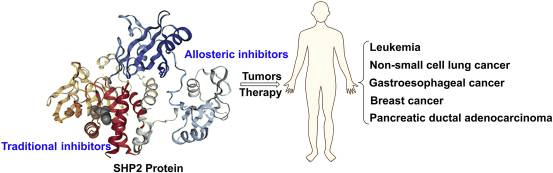
Fig 1. The SHP2 inhibitors in tumor-targeted therapies [1].
Drug resistance in cancer therapy is a major obstacle to achieving successful treatment outcomes. Cancer cells can develop resistance to anticancer drugs, leading to treatment failure and disease progression. This resistance can occur through several mechanisms, including decreased drug uptake, increased drug efflux, altered drug targets, activation of alternative pathways, and alterations in drug metabolism. These mechanisms can result from genetic changes within cancer cells or from interactions with the tumor microenvironment. Understanding the mechanisms of drug resistance is crucial in developing effective cancer therapies that can overcome or prevent resistance and improve patient outcomes.
SHP2 and its role in cancer
SHP2 (Src homology region 2 domain-containing phosphatase-2), also known as PTPN11 (protein tyrosine phosphatase non-receptor type 11), is a protein tyrosine phosphatase that plays a critical role in cellular signaling pathways. SHP2 is the only proven proto-oncoprotein in the PTP family, and its activating mutation or upregulation can lead to the occurrence of various solid tumors such as leukemia, lung adenocarcinoma, colon cancer, breast cancer, neuroblastoma and melanoma. Abnormally activated SHP2 can synergize with receptor tyrosine kinases RTKs and KRAS signaling pathways to promote tumor cell growth, and it is also a downstream sensor of PD-1 signaling, which can inhibit the immune response of T cells to tumors. Therefore, inhibiting SHP2 can not only inhibit the growth of cancer cells, but also activate the anti-tumor effect of the immune system.
SHP2 is involved in multiple signaling pathways that promote tumor growth and survival, such as the RAS-MAPK, PI3K-AKT, and JAK-STAT pathways. SHP2 also regulates the tumor microenvironment by influencing immune cell function and the secretion of cytokines and growth factors that promote tumor growth and survival. Moreover, SHP2 plays a role in cancer stem cell maintenance and tumor metastasis.
Given its role in cancer, SHP2 has emerged as a potential therapeutic target. Inhibiting SHP2 activity has been shown to have anti-tumor effects in preclinical models of cancer. However, the development of SHP2 inhibitors has been challenging due to the complexity of the protein and the need for specificity and selectivity. Nevertheless, several SHP2 inhibitors have been developed and are currently being evaluated in preclinical and clinical studies.
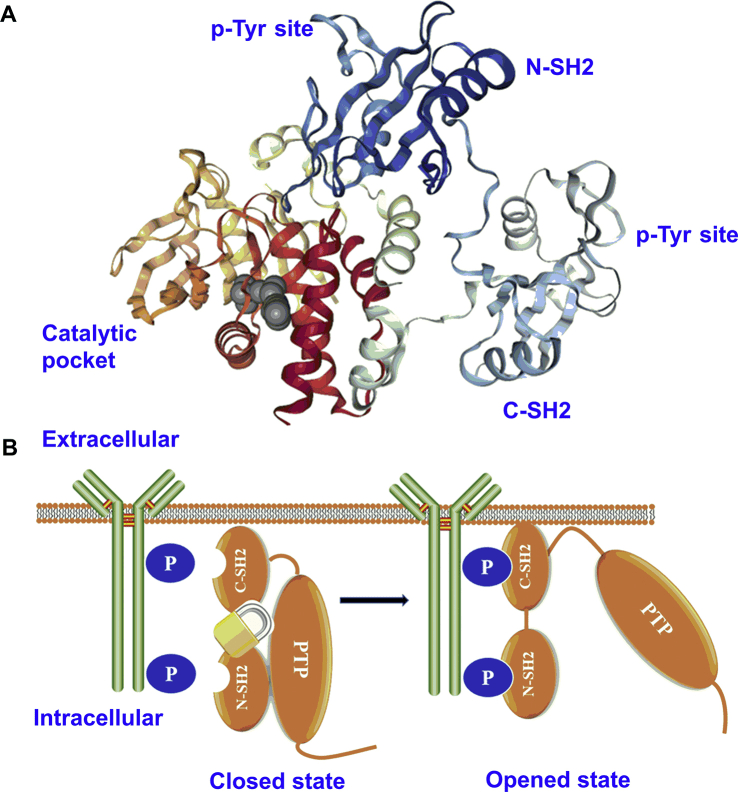
Fig 2. The structure of SHP2 and diagram of SHP2 activation. (A) Crystal structure of the full-length SHP2 (PDB: 2SHP). (B) At closed state, SHP2 is auto-inhibited by N-SH2 domain binding to PTP domain and at an opened state, tyrosine phosphorylation motifs bind to SH2 domains of SHP2, resulting in allosteric regulation and released PTP catalytic activity [1].
The mechanism action of SHP2
SHP2, also known as protein tyrosine phosphatase non-receptor type 11 (PTPN11), is a signaling protein that plays a critical role in several signaling pathways involved in cell growth, differentiation, and survival.
One of the most well-characterized pathways involving SHP2 is the RAS-ERK MAP kinase pathway, which is activated by growth factor signaling and plays a key role in cell proliferation and survival. SHP2 acts as a positive regulator of this pathway by promoting the activation of RAS and downstream signaling through the MAP kinase cascade.
SHP2 is also involved in the PI3K-AKT pathway, which regulates cell survival, metabolism, and proliferation. SHP2 activates this pathway by dephosphorylating inhibitory tyrosine residues on the p85 regulatory subunit of PI3K, leading to activation of AKT.
Furthermore, SHP2 plays a role in the JAK-STAT pathway, which is involved in cytokine signaling and immune responses. SHP2 positively regulates this pathway by dephosphorylating inhibitory tyrosine residues on the JAK kinases, leading to activation of STAT transcription factors.
Finally, SHP2 has been shown to regulate several other signaling pathways, including the Wnt/β-catenin pathway and the TGF-β signaling pathway, which are involved in cell differentiation, migration, and invasion.
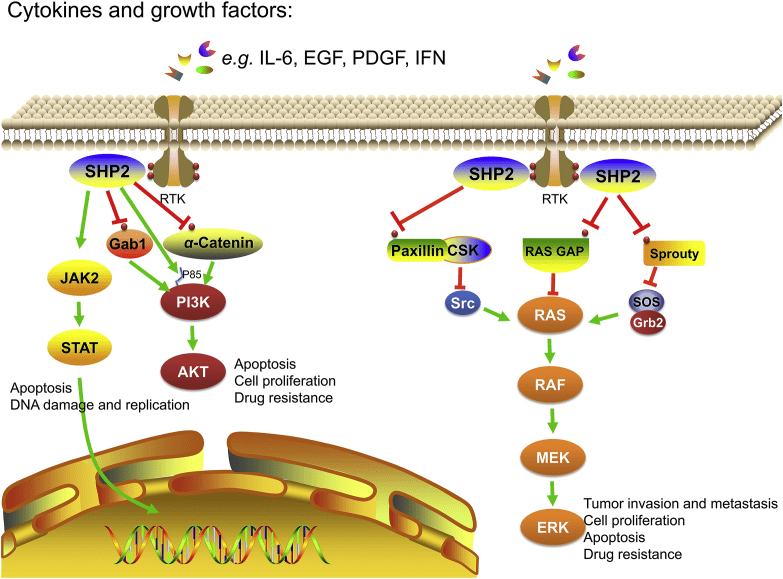
Fig 3. Schematic diagram of SHP2 related cytokines and growth factor dependent RAS-RAF-ERK, PI3K-AKT and JAK-STAT signaling pathways. SHP2 dephosphorylates negative regulators in RAS-RAF-ERK pathway through a variety of mechanisms, such as RAS-GAP, paxillin and sprouty, to regulate signal transduction, tumor invasion, cell proliferation, differentiation, apoptosis and survival; SHP2 inhibits the activation of PI3K-AKT pathway and regulates cell proliferation and apoptosis through dephosphorylation of α-catenin, Gab1 and P85 binding sites; SHP2 dually regulates the JAK-STAT signaling pathway, which is essential for regulating DNA damage, cell growth, differentiation, survival and death [3].
The potential of SHP2 inhibitors as cancer therapeutics
SHP2 inhibitors have emerged as a promising strategy for cancer therapy due to the critical role of SHP2 in promoting tumor growth, survival, and drug resistance. Preclinical studies have demonstrated that SHP2 inhibition can lead to anti-tumor effects in various cancer types, both as a monotherapy and in combination with other therapies, such as chemotherapy and immunotherapy.
Moreover, SHP2 inhibitors have shown good safety profiles in preclinical studies and early clinical trials. Several SHP2 inhibitors have been developed and are currently in clinical trials for the treatment of various types of cancer, including solid tumors and hematologic malignancies.
However, the development of SHP2 inhibitors still faces challenges, including the need for improved specificity and selectivity, as well as the potential for off-target effects. Further research is needed to optimize the pharmacological properties of SHP2 inhibitors and to identify patients who are likely to benefit from this therapy. Nevertheless, SHP2 inhibitors hold great promise as a new class of cancer therapeutics, and their development represents an exciting avenue for improving cancer treatment outcomes.
The different types of SHP2 inhibitors
Several types of SHP2 inhibitors have been developed and are being investigated for their potential as cancer therapeutics. One type of inhibitor is the allosteric inhibitor, which binds to a specific site on SHP2 to prevent its activation. Allosteric inhibitors have shown promising anti-tumor activity in preclinical studies and are currently in clinical trials. Another type of inhibitor is the substrate-trapping inhibitor, which binds to SHP2 and traps its substrates, preventing downstream signaling. These inhibitors have shown potent anti-tumor effects in preclinical studies, particularly in cells with RAS mutations, which are often resistant to other therapies. Furthermore, small molecule inhibitors targeting the catalytic activity of SHP2 have also been developed, although these inhibitors have shown some limitations in terms of selectivity and efficacy. In addition, several natural compounds, such as celastrol and curcumin, have been identified as potential SHP2 inhibitors and are being investigated for their anti-tumor effects.
Overall, SHP2 inhibitors have shown promising preclinical and early clinical results as cancer therapeutics. However, the development of more specific and effective inhibitors is still ongoing, and further research is needed to optimize their pharmacological properties and identify the patient populations most likely to benefit from this therapy.
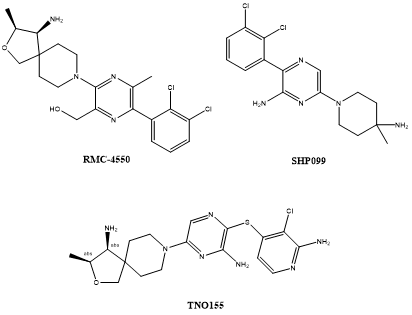
Fig 4. The structure of RMC-4550、SHP099 and TNO155.
RMC-4550
RMC-4550 is an allosteric inhibitor of SHP2, a tyrosine phosphatase protein that is involved in several signaling pathways implicated in cancer development and progression. In a study published in Nature Cell Biology in 2018, Nichols et al. demonstrated that the activity of SHP2 is dependent on the nucleotide cycling of RAS proteins, which are frequently mutated in cancer.
RMC-4550 has shown promising preclinical results as a cancer therapeutic, particularly in preclinical models of KRAS-mutant tumors. In a phase I clinical trial, RMC-4550 demonstrated favorable pharmacokinetics and pharmacodynamics, and early efficacy signals were observed in patients with KRAS-mutant lung adenocarcinoma and colorectal cancer.
In a phase I/II clinical trial, RMC-4550 is currently being evaluated in combination with the MEK inhibitor cobimetinib for the treatment of patients with KRAS-mutant non-small cell lung cancer (NSCLC). The trial is designed to assess the safety and efficacy of the combination therapy in patients who have progressed on prior treatment.
Overall, RMC-4550 represents a promising therapy for cancers driven by RAS mutations, which have historically been difficult to treat. Further clinical trials will be needed to fully assess the safety and efficacy of RMC-4550 as a cancer therapeutic, but the early results are encouraging.
SHP099
SHP099 is a potent and selective small-molecule inhibitor of SHP2. It was developed by a team of researchers from the Novartis Institute for Biomedical Research and was reported in the journal Nature in 2016.
In preclinical studies, SHP099 was found to effectively inhibit the growth of cancer cells with aberrant activation of the RAS-MAPK signaling pathway. This pathway is frequently dysregulated in various cancer types, including lung, pancreatic, and colorectal cancers. By inhibiting SHP2, SHP099 reduces the activity of downstream signaling molecules in this pathway, leading to decreased cell proliferation and survival.
SHP099 has shown promising results in mouse models of human cancer, both as a monotherapy and in combination with other agents. Notably, it has been shown to be effective against tumors that are resistant to other targeted therapies, such as EGFR inhibitors.
In addition to its potential as an anticancer agent, SHP099 has also been investigated for its therapeutic potential in other diseases, such as Noonan syndrome and neurofibromatosis type 1. These diseases are caused by genetic mutations that lead to dysregulated signaling through the RAS-MAPK pathway, and SHP2 is a key mediator of his pathway.
Overall, SHP099 represents a promising therapeutic option for cancers with dysregulated RAS-MAPK signaling, and its development highlights the potential of targeting SHP2 as a novel approach to cancer treatment.
TNO155
TNO155 is a highly selective, potent, and allosteric inhibitor of SHP2, which has emerged as a promising target for cancer therapy due to its critical role in several cancer signaling pathways. The development of TNO155 was based on the optimization of a series of small-molecule inhibitors of SHP2, resulting in the identification of a compound with favorable pharmacological properties and efficacy in preclinical models of cancer.
Studies have shown that TNO155 inhibits the activity of SHP2 in cancer cells with various mutations, including those in KRAS, BRAF, and FGFR, which are frequently observed in several types of cancer. TNO155 has been shown to inhibit the downstream signaling pathways of these oncogenes, resulting in the suppression of cancer cell proliferation and survival. Additionally, TNO155 has been demonstrated to sensitize cancer cells to other targeted therapies, including MEK inhibitors and immunotherapies.
The preclinical studies of TNO155 have shown that it has a favorable pharmacokinetic profile and is well-tolerated in animal models. Moreover, TNO155 demonstrated antitumor activity in xenograft models of several types of cancer, including lung, breast, and colon cancer, as well as acute myeloid leukemia.
Currently, TNO155 is undergoing phase 1 clinical trials in patients with advanced solid tumors. The results of the ongoing trials will help determine the safety, pharmacokinetics, and efficacy of TNO155 in humans. If successful, TNO155 could potentially become a promising therapeutic option for cancer patients with oncogenic mutations in SHP2 signaling pathways.
Summary
Cancer drug resistance is a major challenge to effective treatment outcomes. It can result from several mechanisms including changes in drug targets, alterations in drug metabolism, activation of alternative pathways, and interaction with the tumor microenvironment. SHP2 (Src homology region 2 domain-containing phosphatase-2), a protein tyrosine phosphatase, plays a crucial role in cancer development, activation of immune system response to tumors and the regulation of multiple signaling pathways that promote tumor growth and survival. Given its role in cancer, SHP2 inhibitors have emerged as a potential therapeutic target. SHP2 inhibitors have shown great promise as cancer therapeutics due to the role of SHP2 in promoting tumor growth, survival, and drug resistance. Several types of SHP2 inhibitors are being developed, including allosteric inhibitors and substrate-trapping inhibitors, which have shown promising results in preclinical studies and are currently being investigated in clinical trials for the treatment of various cancers. Further research is needed to optimize the pharmacological properties of SHP2 inhibitors and to identify patients who are likely to benefit from this therapy.
Reference
- Song, Z., Wang, M., Ge, Y., Chen, X. P., Xu, Z., Sun, Y., & Xiong, X. F. (2021). Tyrosine phosphatase SHP2 inhibitors in tumor-targeted therapies. Acta pharmaceutica Sinica. B, 11(1), 13–29.
- Liu, Q., Qu, J., Zhao, M., Xu, Q., & Sun, Y. (2020). Targeting SHP2 as a promising strategy for cancer immunotherapy. Pharmacological research, 152, 104595.
- Liu, M., Gao, S., Elhassan, R. M., Hou, X., & Fang, H. (2021). Strategies to overcome drug resistance using SHP2 inhibitors. Acta pharmaceutica Sinica. B, 11(12), 3908–3924.
- Nichols, R. J., Haderk, F., Stahlhut, C., Schulze, C. J., Hemmati, G., Wildes, D., Tzitzilonis, C., Mordec, K., Marquez, A., Romero, J., Hsieh, T., Zaman, A., Olivas, V., McCoach, C., Blakely, C. M., Wang, Z., Kiss, G., Koltun, E. S., Gill, A. L., Singh, M., … Bivona, T. G. (2018). RAS nucleotide cycling underlies the SHP2 phosphatase dependence of mutant BRAF-, NF1- and RAS-driven cancers. Nature cell biology, 20(9), 1064–1073.
- Chen, Y. N., LaMarche, M. J., Chan, H. M., Fekkes, P., Garcia-Fortanet, J., Acker, M. G., Antonakos, B., Chen, C. H., Chen, Z., Cooke, V. G., Dobson, J. R., Deng, Z., Fei, F., Firestone, B., Fodor, M., Fridrich, C., Gao, H., Grunenfelder, D., Hao, H. X., Jacob, J., … Fortin, P. D. (2016). Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature, 535(7610), 148–152.
- LaMarche, M. J., Acker, M., Argintaru, A., Bauer, D., Boisclair, J., Chan, H., Chen, C. H., Chen, Y. N., Chen, Z., Deng, Z., Dore, M., Dunstan, D., Fan, J., Fekkes, P., Firestone, B., Fodor, M., Garcia-Fortanet, J., Fortin, P. D., Fridrich, C., Giraldes, J., … Zhu, S. (2020). Identification of TNO155, an Allosteric SHP2 Inhibitor for the Treatment of Cancer. Journal of medicinal chemistry, 63(22), 13578–13594.




