Small Cell Lung Cancer (SCLC): Understanding Its Biology, Diagnosis, and the Future of Targeted Therapies
Abstract
Small cell lung cancer (SCLC) is an aggressive and rapidly progressing subtype of lung cancer, accounting for approximately 15% of all lung cancer cases. Characterized by a high mutational burden, early metastasis, and poor prognosis, SCLC has remained a challenge in oncology due to its limited treatment advancements over the past decades. This article explores the cellular and molecular mechanisms underlying SCLC, including the loss of TP53 and RB1 tumor suppressor genes, the role of neuroendocrine markers, and emerging biomarker-driven therapies. Traditional treatment approaches, such as platinum-based chemotherapy and radiation therapy, provide only temporary tumor control, as most patients relapse within a year. However, recent breakthroughs in targeted therapy, immunotherapy, and liquid biopsy techniques offer new hope for improved patient outcomes. This article delves into these advances, discussing promising PARP inhibitors, DLL3-targeted antibody-drug conjugates, immune checkpoint inhibitors, and CAR-T cell therapy, all of which are reshaping the treatment landscape for SCLC. Understanding the molecular biology of SCLC is crucial for developing personalized, precision-based treatments that could potentially extend survival and improve quality of life for patients.
Introduction: Understanding Small Cell Lung Cancer (SCLC)
Small cell lung cancer (SCLC) is a highly aggressive and lethal form of lung cancer that accounts for approximately 15% of all lung cancer cases. Despite a decline in incidence due to reduced smoking rates, it remains a significant public health concern due to its rapid progression and poor prognosis. The five-year survival rate for SCLC is alarmingly low, typically less than 7%, due to its high metastatic potential and resistance to therapy. Unlike non-small cell lung cancer (NSCLC), which can often be treated with surgery, SCLC is primarily managed with chemotherapy and radiation, as most patients present with advanced-stage disease at diagnosis.
The aggressive nature of SCLC stems from its distinct biological and molecular characteristics. It is believed to originate from neuroendocrine cells in the lungs, which have the ability to rapidly divide and spread. The disease is characterized by a high mutation rate, primarily driven by long-term exposure to carcinogens like tobacco smoke. Nearly all SCLC cases exhibit inactivation of two crucial tumor suppressor genes: TP53 and RB1, which normally function to regulate cell growth and prevent malignancy. This loss of tumor suppression leads to uncontrolled proliferation and tumor development.
A major challenge in treating SCLC is its tendency to develop resistance to standard therapies. Although initial responses to chemotherapy can be dramatic, relapse occurs quickly, and second-line treatments often yield limited success. The lack of major treatment advancements over the past several decades underscores the urgent need for new therapeutic strategies targeting SCLC-specific molecular pathways.
This blog post will delve deeper into the cellular and molecular basis of SCLC, its diagnostic criteria, and the latest advancements in targeted therapies and immunotherapy. Understanding the biology of SCLC is key to developing innovative treatments that could improve survival rates for patients suffering from this devastating disease.
The Cellular and Molecular Basis of SCLC: How It Develops
Small cell lung cancer (SCLC) is a distinct and aggressive form of lung cancer that originates from neuroendocrine cells in the lung. These cells possess hormone-producing capabilities and play a crucial role in lung homeostasis. However, exposure to carcinogens, particularly tobacco smoke, triggers a series of genetic mutations that transform these neuroendocrine cells into highly proliferative cancerous cells.
A hallmark of SCLC is the nearly universal inactivation of the TP53 and RB1 tumor suppressor genes, both of which play essential roles in maintaining genomic stability and controlling the cell cycle. Their loss results in unchecked cellular proliferation, leading to rapid tumor growth and metastasis. Additionally, the MYC oncogene is frequently amplified in aggressive subtypes of SCLC, further driving tumor progression.
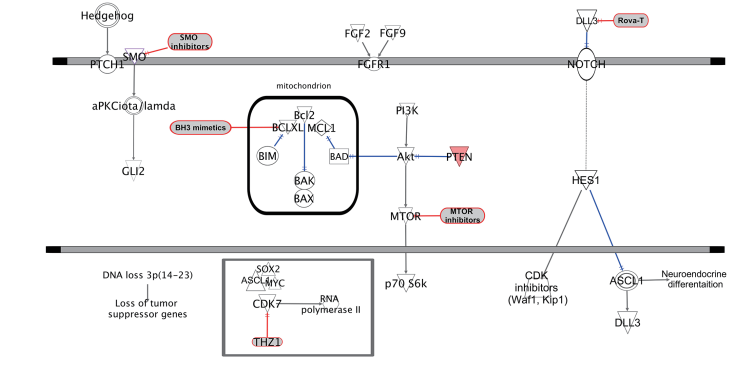
Figure 1. Potential therapeutic targets in SCLC. SCLC, small cell lung cancer.
Another key pathway involved in SCLC is the NOTCH signaling pathway, which plays a dual role in tumorigenesis. While NOTCH activation can act as a tumor suppressor in neuroendocrine cancers, many SCLC tumors display NOTCH inactivating mutations, leading to enhanced tumor growth . The ASCL1 and NEUROD1 transcription factors also contribute to tumor development, with ASCL1 playing a key role in maintaining neuroendocrine differentiation, while NEUROD1 promotes an aggressive, metastasis-prone phenotype.
Understanding these molecular mechanisms is crucial for developing targeted therapies aimed at blocking these oncogenic pathways, thereby slowing or preventing disease progression.
Diagnosis and Histological Features of SCLC
Diagnosing small cell lung cancer (SCLC) requires a combination of imaging, histopathology, and molecular testing. Because SCLC presents aggressively, early detection is rare, and most patients are diagnosed at advanced stages.
Histological Characteristics of SCLC
SCLC is classified as a high-grade neuroendocrine carcinoma, characterized by:
Small, round tumor cells with high nuclear-to-cytoplasmic ratio.
High mitotic index (>50–70% Ki-67 staining).
Frequent necrosis, often extensive.
Nuclear molding, where tumor cells are closely packed together.
Due to its small, undifferentiated appearance, SCLC can resemble other neuroendocrine tumors or even lymphomas, making immunohistochemistry (IHC) a crucial tool for diagnosis.
Immunohistochemical Markers for SCLC
To confirm an SCLC diagnosis, pathologists rely on neuroendocrine markers, including:
CD56 – Found in nearly all SCLC cases but lacks specificity.
Chromogranin A & Synaptophysin – Indicators of neuroendocrine differentiation.
TTF-1 (Thyroid Transcription Factor-1) – Present in 70–90% of SCLC cases.
Differentiating SCLC from Other Lung Neuroendocrine Tumors
SCLC exists on a spectrum of lung neuroendocrine tumors, which also includes large cell neuroendocrine carcinoma (LCNEC), atypical carcinoids, and typical carcinoids. While SCLC and LCNEC share some genetic similarities (such as RB1 and TP53 loss), carcinoids arise from different progenitor cells and tend to have lower proliferation rates and distinct molecular profiles.
Understanding the histological and molecular characteristics of SCLC is critical for ensuring accurate diagnosis and guiding appropriate treatment strategies, particularly as targeted therapies and immunotherapies emerge.
Emerging Treatments and Targeted Therapies for SCLC
Small cell lung cancer (SCLC) remains one of the most challenging malignancies to treat due to its rapid progression and high relapse rate. Although traditional chemotherapy and radiation therapy have been the mainstays of treatment for decades, emerging targeted therapies and immunotherapies offer hope for improved patient outcomes.
Current Standard Treatments for SCLC
Platinum-based chemotherapy (Cisplatin or Carboplatin with Etoposide) – The first-line treatment for SCLC, but most patients relapse within 6–12 months.
Radiation therapy – Used in limited-stage SCLC to improve survival when combined with chemotherapy.
Prophylactic Cranial Irradiation (PCI) – Reduces the risk of brain metastases in patients who respond to initial treatment.
New and Emerging Drug Targets in SCLC
DLL3-Targeted Therapy (Rovalpituzumab Tesirine – Rova-T)
DLL3 (Delta-like protein 3) is expressed in over 80% of SCLC tumors but not in normal lung tissue, making it an attractive drug target.
Rova-T is an antibody-drug conjugate (ADC) designed to target and destroy DLL3-positive SCLC cells. Early clinical trials showed promise, but further studies are needed to improve efficacy.
PARP Inhibitors (Talazoparib, Olaparib, Veliparib)
SCLC tumors exhibit defects in DNA repair mechanisms, making them susceptible to PARP inhibitors, which block DNA damage repair and promote tumor cell death.
Ongoing phase II and III clinical trials are evaluating the role of PARP inhibitors in relapsed SCLC.
EZH2 Inhibitors
EZH2 (Enhancer of Zeste Homolog 2) plays a role in epigenetic regulation, and its overexpression in SCLC has been linked to tumor proliferation.
Experimental drugs targeting EZH2 are currently being tested in clinical trials.
Hedgehog Signaling Inhibitors
The Hedgehog signaling pathway has been implicated in SCLC tumor progression and recurrence.
Drugs that inhibit Hedgehog signaling, such as Vismodegib, have shown potential to slow tumor growth in preclinical studies.
Immunotherapy (Checkpoint Inhibitors: PD-1/PD-L1 and CTLA-4 Blockade)
Given SCLC’s high mutational burden, checkpoint inhibitors like Nivolumab, Atezolizumab, and Ipilimumab are being studied for enhancing anti-tumor immune responses.
Recent studies have shown that patients with high PD-L1 expression respond better to immune checkpoint inhibitors.
Future Outlook and Conclusion: The Path Ahead for SCLC Research
Despite significant progress in understanding the molecular mechanisms of small cell lung cancer (SCLC), its prognosis remains poor. However, new advances in targeted therapy, immunotherapy, and precision medicine are shaping a hopeful future for SCLC treatment.
Challenges in Treating SCLC
High tumor heterogeneity – SCLC tumors evolve rapidly, making them resistant to single-agent therapies.
Limited biopsy availability – Many SCLC patients have metastatic disease at diagnosis, limiting access to tumor tissue for molecular profiling.
Rapid recurrence after treatment – Most patients experience disease relapse within a year of initial therapy.
What’s Next in SCLC Research?
Liquid Biopsies and Circulating Tumor Cells (CTCs)
Since SCLC is highly metastatic, blood-based liquid biopsies analyzing CTCs may help detect recurrence and guide personalized treatment.
Combination Therapies
Emerging research suggests that combining immunotherapy with chemotherapy or targeted therapy could extend survival in SCLC patients.
Clinical trials are ongoing to explore checkpoint inhibitors + PARP inhibitors as a potential dual approach for treating resistant SCLC.
CAR-T Cell Therapy for SCLC
Chimeric Antigen Receptor (CAR)-T therapy is a promising approach targeting SCLC-specific markers such as DLL3 and CD56.
Early trials are exploring whether CAR-T therapy can effectively eliminate SCLC tumor cells while avoiding severe side effects.
Final Thoughts
While SCLC remains one of the most difficult lung cancers to treat, recent discoveries in genetics, immunotherapy, and targeted therapy offer new hope for patients. Precision medicine and biomarker-driven therapies may finally help break the decades-long barrier of treatment resistance in SCLC.
The future of SCLC treatment lies in personalized approaches, integrating molecular profiling, targeted therapy, and immunotherapy to improve patient survival and quality of life.
References
American Cancer Society. (2023). Lung cancer statistics and facts. Retrieved from https://www.cancer.org
George, J., Lim, J. S., Jang, S. J., et al. (2015). Comprehensive genomic profiles of small cell lung cancer. Nature, 524(7563), 47-53. https://doi.org/10.1038/nature14664
Karachaliou, N., Pilotto, S., Lazzari, C., et al. (2016). Cellular and molecular biology of small cell lung cancer: An overview. Translational Lung Cancer Research, 5(1), 2-15. https://doi.org/10.3978/j.issn.2218-6751.2016.01.02
Pleasance, E. D., Stephens, P. J., O’Meara, S., et al. (2010). A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature, 463(7278), 184-190. https://doi.org/10.1038/nature08629
Borromeo, M. D., Savage, T. K., Kollipara, R. K., He, M., Augustyn, A., Osborne, J. K., … & Johnson, J. E. (2016). ASCL1 and NEUROD1 reveal heterogeneity in pulmonary neuroendocrine tumors. Cancer Research, 76(16), 4760-4770. https://doi.org/10.1158/0008-5472.CAN-15-3453
George, J., Lim, J. S., Jang, S. J., et al. (2015). Comprehensive genomic profiles of small cell lung cancer. Nature, 524(7563), 47-53. https://doi.org/10.1038/nature14664
Peifer, M., Fernández-Cuesta, L., Sos, M. L., et al. (2012). Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nature Genetics, 44(10), 1104-1110. https://doi.org/10.1038/ng.2396
Hellmann, M. D., Callahan, M. K., Awad, M. M., et al. (2018). Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell, 33(5), 853-861. https://doi.org/10.1016/j.ccell.2018.04.001
Jensen, M. C., & Berthold, F. (2007). Targeting the neural cell adhesion molecule in cancer. Cancer Letters, 258(1), 9-21. https://doi.org/10.1016/j.canlet.2007.07.002
Owonikoko, T. K., Zhang, G., Deng, X., et al. (2014). Poly (ADP) ribose polymerase enzyme inhibitor, veliparib, potentiates chemotherapy and radiation in vitro and in vivo in small cell lung cancer. Cancer Medicine, 3(6), 1579-1594. https://doi.org/10.1002/cam4.323
Saunders, L. R., Bankovich, A. J., Anderson, W. C., et al. (2015). A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Science Translational Medicine, 7(302), 302ra136. https://doi.org/10.1126/scitranslmed.aac9459