Sotorasib: A New Treatment Option for NSCLC Patients with KRAS G12C Mutation
Abstract
Sotorasib, also known as AMG 510, is a drug designed to treat non-small cell lung cancer (NSCLC). It works as a small molecule inhibitor of KRAS G12C, a mutated form of the KRAS gene that is found in around 13% of NSCLC patients. KRAS G12C plays a role in the growth and spread of cancer cells, and sotorasib works by blocking its activity. The drug was granted approval by the US FDA in May 2021 for use in treating NSCLC patients with the KRAS G12C mutation. Sotorasib offers a promising new treatment option for this subset of lung cancer patients.
NSCLC and KRAS G12C mutation
Non-small cell lung cancer (NSCLC) is a type of lung cancer that accounts for around 85% of all lung cancer cases. It typically grows and spreads more slowly than small cell lung cancer, but can still be difficult to treat if it has advanced or metastasized to other parts of the body. One specific genetic mutation that has been identified in NSCLC is the KRAS G12C mutation. KRAS is a gene that plays a critical role in cell signaling pathways that regulate cell growth and differentiation. When the KRAS gene is mutated, it can lead to abnormal cell growth and division, which can ultimately lead to cancer. The KRAS G12C mutation is a specific mutation that occurs in the KRAS gene, where a guanine (G) is replaced by a cytosine (C) at position 12 of the KRAS protein.
This mutation is found in around 13% of NSCLC cases and is associated with poor prognosis and limited treatment options. Historically, therapies targeting KRAS mutations have been largely unsuccessful due to the difficulty of inhibiting the KRAS protein, which has been described as “undruggable.” However, recent advancements in drug development have led to the discovery of small molecule inhibitors that are capable of selectively targeting the KRAS G12C mutation.
One such inhibitor is Sotorasib (also known as AMG 510), which was granted FDA approval in May 2021 for the treatment of NSCLC patients with the KRAS G12C mutation. Sotorasib works by blocking the activity of the mutated KRAS protein, which prevents abnormal cell growth and division. It offers a promising new treatment option for NSCLC patients with this specific genetic mutation, who previously had limited treatment options available.
The approval of Sotorasib is a significant development in the treatment of NSCLC, as it represents the first targeted therapy specifically approved for KRAS G12C-mutant NSCLC. It is also a promising example of the potential of precision medicine, which involves tailoring treatment to the specific genetic characteristics of a patient’s tumor. With continued research and development, it is hoped that additional targeted therapies will be discovered for other mutations associated with NSCLC, ultimately leading to improved outcomes for patients with this challenging disease.
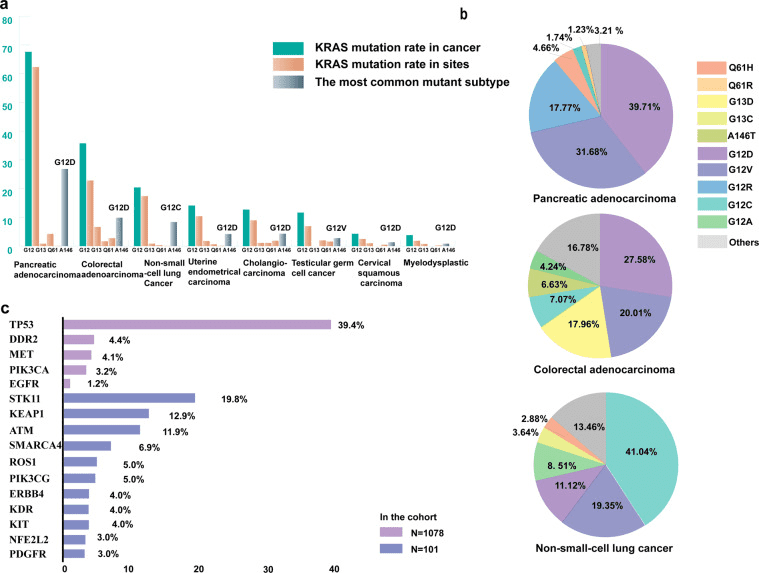
Fig 1. KRAS mutation in cancer [1].
The mechanism action of Sotorasib
Sotorasib as a small molecule inhibitor that selectively targets the KRAS G12C mutation found in non-small cell lung cancer (NSCLC) and other cancers. KRAS G12C is a specific genetic mutation that occurs in the KRAS gene, which leads to abnormal cell growth and division, ultimately resulting in cancer. Sotorasib works by inhibiting the activity of the mutated KRAS protein, preventing abnormal cell growth and division. It does this by binding to the KRAS protein at the site of the G12C mutation, which prevents it from activating downstream signaling pathways that are critical for cell growth and survival.
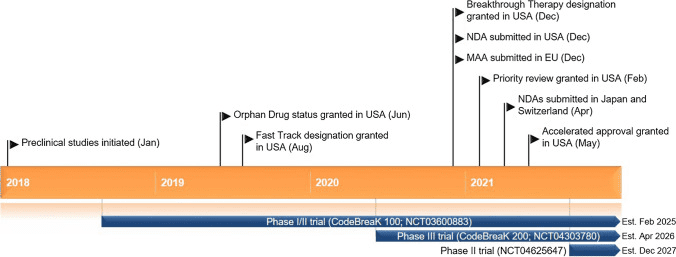
Fig 2. Key milestones in the development of sotorasib for the treatment of KRAS G12C-mutated non-small cell lung cancer. MAA Market Authorisation Application, NDA New Drug Application [2].
The preclinical studies and clinical trial
Preclinical studies have shown promising results for sotorasib. In one study published in Cancer Discovery, sotorasib was tested in NSCLC cell lines and mouse models that harbored the KRAS G12C mutation. The study found that sotorasib effectively inhibited the activity of the KRAS G12C protein, resulting in decreased cell proliferation and increased apoptosis (cell death) in the NSCLC cell lines. In the mouse models, treatment with sotorasib led to tumor regression and prolonged survival.
Another preclinical study published in Nature Communications evaluated the efficacy of sotorasib in combination with other targeted therapies. The study found that combining sotorasib with inhibitors of the MEK and ERK signaling pathways (which are downstream of KRAS) resulted in enhanced tumor growth inhibition in mouse models of NSCLC and colorectal cancer.
In addition to these preclinical studies, sotorasib has also been evaluated in clinical trials. In a phase I/II trial, sotorasib was tested in patients with advanced solid tumors, including NSCLC with KRAS G12C mutation. The trial found that sotorasib was well-tolerated, with manageable side effects, and demonstrated promising anti-tumor activity in patients with NSCLC.
Based on the results of these preclinical and clinical studies, sotorasib was granted accelerated FDA approval in May 2021 for the treatment of NSCLC patients with KRAS G12C mutation who have received at least one prior systemic therapy. It is the first targeted therapy specifically approved for this patient population, and offers a promising new treatment option for NSCLC patients with this genetic mutation.
In summary, sotorasib is a small molecule inhibitor that selectively targets the KRAS G12C mutation found in NSCLC and other cancers. Preclinical studies have shown promising results, including decreased cell proliferation and increased apoptosis in NSCLC cell lines, as well as tumor regression and prolonged survival in mouse models. Clinical trials have demonstrated promising anti-tumor activity and tolerability in patients with advanced solid tumors, including NSCLC with KRAS G12C mutation. The approval of sotorasib represents a significant development in the treatment of NSCLC, and offers hope for patients with this challenging disease.
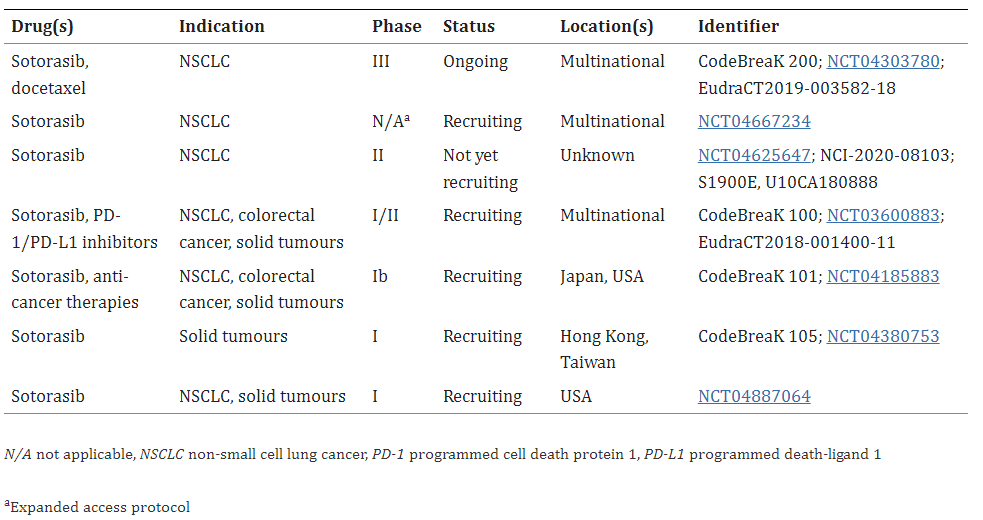
Table 1. Key clinical trials of sotorasib (Amgen) [3].
Comparison to other therapies
In addition to sotorasib, there are other targeted therapies currently being developed for KRAS-mutant NSCLC, including inhibitors of the MEK and ERK signaling pathways. While these therapies have shown promising results in preclinical studies and clinical trials, none have yet received FDA approval for the treatment of NSCLC with KRAS G12C mutation.
In terms of efficacy and safety, sotorasib has shown promising results in clinical trials, with a high response rate and manageable side effects. However, direct comparisons to other therapies are difficult to make, as each therapy targets different aspects of the KRAS signaling pathway and may have different efficacy and safety profiles. Overall, sotorasib represents an important new treatment option for NSCLC patients with KRAS G12C mutation, and further research is needed to fully understand its place among other targeted therapies for this disease.
Conclusion and future directions
Sotorasib is a targeted therapy that has shown promising results in the treatment of NSCLC with KRAS G12C mutation. It works by inhibiting the activity of the mutant KRAS protein, which is a key driver of cancer growth in this population of patients. Sotorasib has demonstrated a high response rate and manageable side effects in clinical trials, making it an important new treatment option for NSCLC patients with KRAS G12C mutation.
The approval of sotorasib by the FDA represents a significant step forward in the treatment of KRAS-mutant NSCLC, which has historically been difficult to treat with targeted therapies. Future research directions include investigating the optimal sequencing and combination of sotorasib with other targeted therapies and immunotherapies, as well as identifying potential biomarkers of response to sotorasib.
Overall, sotorasib has the potential to significantly impact the treatment landscape for NSCLC patients with KRAS G12C mutation, and ongoing research will continue to refine its role in the management of this disease.
Reference
- Huang, L., Guo, Z., Wang, F., & Fu, L. (2021). KRAS mutation: from undruggable to druggable in cancer. Signal transduction and targeted therapy, 6(1), 386.
- Skoulidis, F., Li, B. T., Dy, G. K., Price, T. J., Falchook, G. S., Wolf, J., Italiano, A., Schuler, M., Borghaei, H., Barlesi, F., Kato, T., Curioni-Fontecedro, A., Sacher, A., Spira, A., Ramalingam, S. S., Takahashi, T., Besse, B., Anderson, A., Ang, A., Tran, Q., … Govindan, R. (2021). Sotorasib for Lung Cancers with KRAS p.G12C Mutation. The New England journal of medicine, 384(25), 2371–2381.
- Blair H. A. (2021). Sotorasib: First Approval. Drugs, 81(13), 1573–1579.
- Hong, D. S., Fakih, M. G., Strickler, J. H., Desai, J., Durm, G. A., Shapiro, G. I., Falchook, G. S., Price, T. J., Sacher, A., Denlinger, C. S., Bang, Y. J., Dy, G. K., Krauss, J. C., Kuboki, Y., Kuo, J. C., Coveler, A. L., Park, K., Kim, T. W., Barlesi, F., Munster, P. N., … Li, B. T. (2020). KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. The New England journal of medicine, 383(13), 1207–1217.
- Strickler, J. H., Satake, H., George, T. J., Yaeger, R., Hollebecque, A., Garrido-Laguna, I., Schuler, M., Burns, T. F., Coveler, A. L., Falchook, G. S., Vincent, M., Sunakawa, Y., Dahan, L., Bajor, D., Rha, S. Y., Lemech, C., Juric, D., Rehn, M., Ngarmchamnanrith, G., Jafarinasabian, P., … Hong, D. S. (2023). Sotorasib in KRAS p.G12C-Mutated Advanced Pancreatic Cancer. The New England journal of medicine, 388(1), 33–43.
- Nakajima, E. C., Drezner, N., Li, X., Mishra-Kalyani, P. S., Liu, Y., Zhao, H., Bi, Y., Liu, J., Rahman, A., Wearne, E., Ojofeitimi, I., Hotaki, L. T., Spillman, D., Pazdur, R., Beaver, J. A., & Singh, H. (2022). FDA Approval Summary: Sotorasib for KRAS G12C-Mutated Metastatic NSCLC. Clinical cancer research: an official journal of the American Association for Cancer Research, 28(8), 1482–1486.
- Canon, J., Rex, K., Saiki, A. Y., Mohr, C., Cooke, K., Bagal, D., Gaida, K., Holt, T., Knutson, C. G., Koppada, N., Lanman, B. A., Werner, J., Rapaport, A. S., San Miguel, T., Ortiz, R., Osgood, T., Sun, J. R., Zhu, X., McCarter, J. D., Volak, L. P., … Lipford, J. R. (2019). The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature, 575(7781), 217–223.
- FDA Approves First KRAS Inhibitor: Sotorasib. (2021). Cancer discovery, 11(8), OF4.