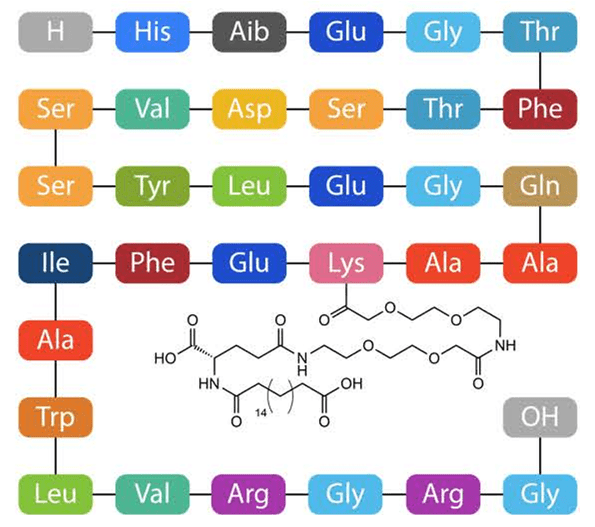
Summary of the synthetic route of Oseltamivir (Tamiflu)
Abstract
The paper traces the evolution of Oseltamivir (Tamiflu) synthesis, from Gilead’s initial methods in the 1990s to Roche’s current industrial route. Early challenges included raw material shortages and safety risks with azide intermediates. Roche’s 12-step method, still the most widely used, has faced issues with scalability and safety. Over time, alternative azide-free and non-shikimic acid methods have emerged, improving yields and cost-efficiency. Key advancements like continuous flow chemistry and optimized azide reactions show promise, though the Roche route remains dominant. Ongoing research aims to address challenges related to scalability, safety, and environmental impact in Tamiflu production.
1. Background
Oseltamivir (Tamiflu) was discovered by Gilead in 1995, patented in 1996, subsequently transferred to Roche, and approved by the FDA in 1999. Tamiflu remains the most commonly used anti-influenza virus drug. This review, "The evolution of Tamiflu synthesis, 20 years on: Advent of enabling technologies the last piece of the puzzle?", introduces the evolution of Tamiflu to efficient and safe synthetic routes since its first approval 20 years ago. More than 70 synthetic routes have been reported so far.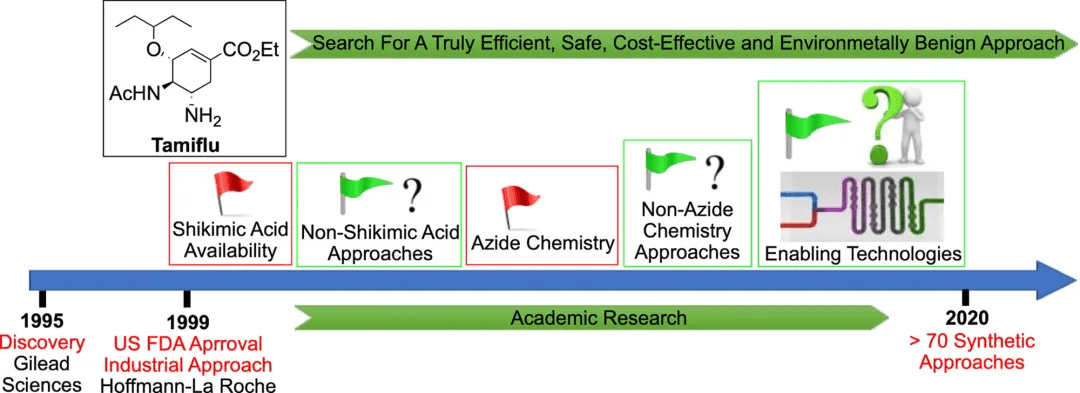
Figure 1. Evolution of the Tamiflu synthesis route.
Gilead's first Tamiflu synthesis route
2.1 Laboratory route
(−)-Shikimic acid methyl ester derivative 3 was treated under Mitsunobu conditions to selectively activate the least hindered hydroxyl group at C-5, while the hydroxyl group at C-3 was protected by MOM to give epoxide 4. The epoxide was then opened with sodium azide to give molecule 5 , which was reduced to give aziridine 6 . Sodium azide was used again for selective aziridine ring opening, and MOM deprotection gave 7. A two-step one-pot synthesis of trityl-protected aziridine 8 was performed. Under Lewis acid catalysis, 3-pentanol reacted with aziridine 8 for selective ring opening, followed by amine acetylation, reduction of the azide group to the amino group, and finally hydrolysis under alkaline conditions to give Tamiflu API (molecule 2). A total of 14 steps with an overall yield of 15%, but this route could not be scaled up at the time due to the availability of shikimic acid and the potential explosion hazard of the azide intermediate.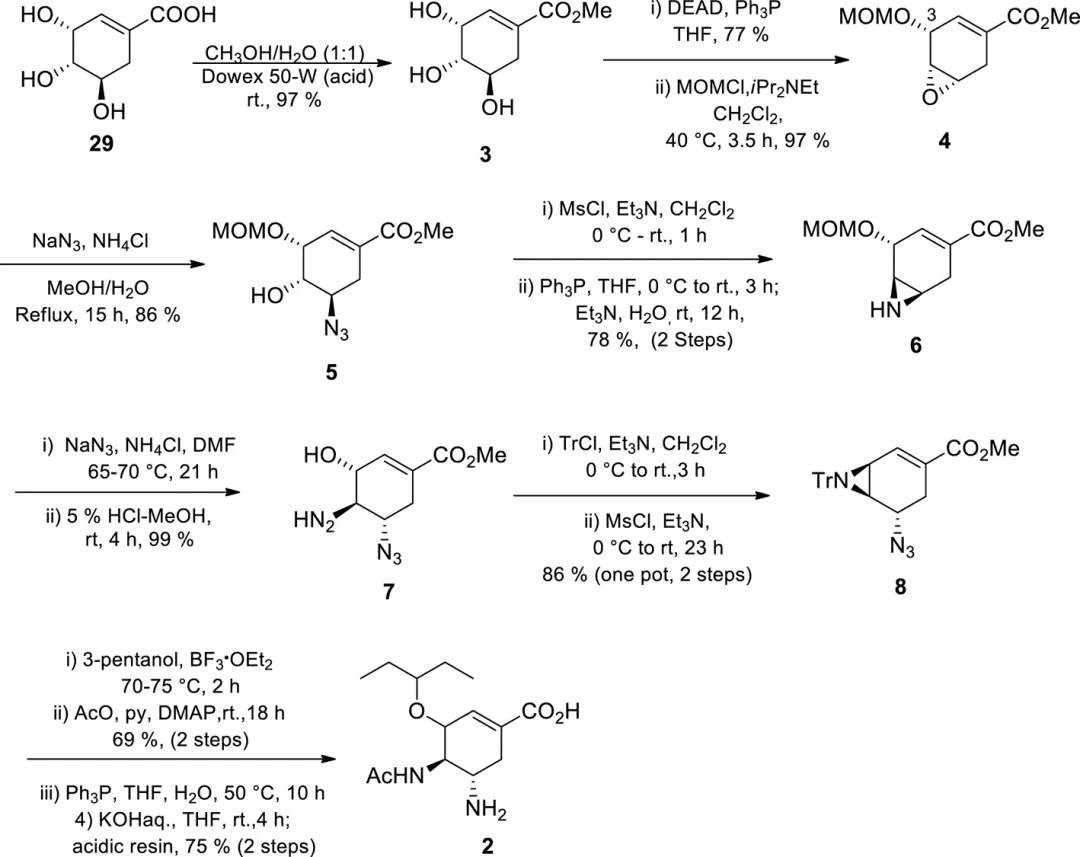
Figure 2. Gilead's first synthesis of Tamiflu.
2.2 Pilot plant route
Due to the large shortage of shikimic acid at the time, Gilead scientists hoped to prepare Tamiflu on a large scale from the more available (−)-quinic acid 9. This route has 12 steps with an overall yield of 4.4%. Despite the low yield, Tamiflu was successfully synthesized in a kg-scale pilot plant and the azide was safely handled. In addition, this route uses minimal protecting group manipulation and does not require chromatographic separation.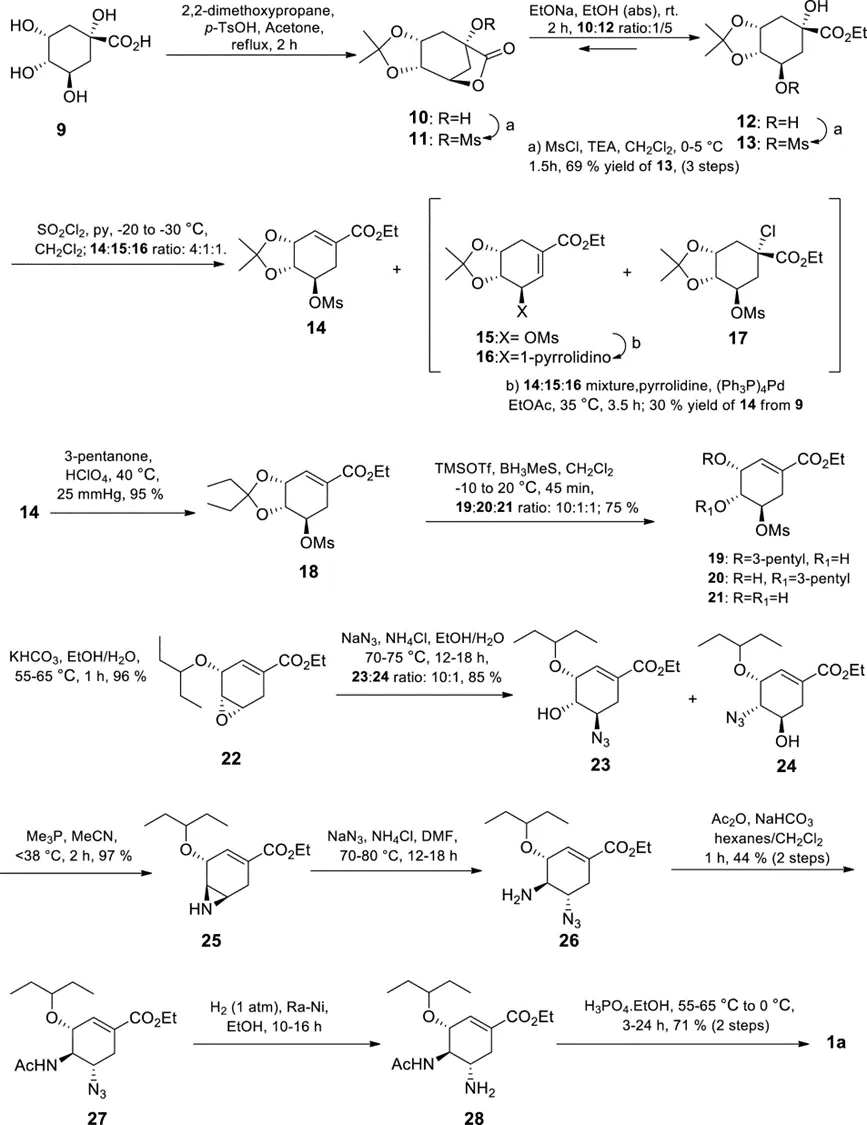
Figure 3. Gilead’s first large-scale synthesis of Tamiflu. 1a is the phosphate salt of 2.
3 . Roche's industrial synthesis route
Later, Gilead and Roche collaborated to improve the availability of (−)-shikimic acid and developed an efficient and safe industrial synthesis route, which is currently the only industrial synthesis route for Tamiflu, with a total of 12 steps and a yield of 35%. Different from Gilead's route, the Roche route introduces the 3-pentyloxy moiety at the early C-3 by regioselective reduction of acetal 18.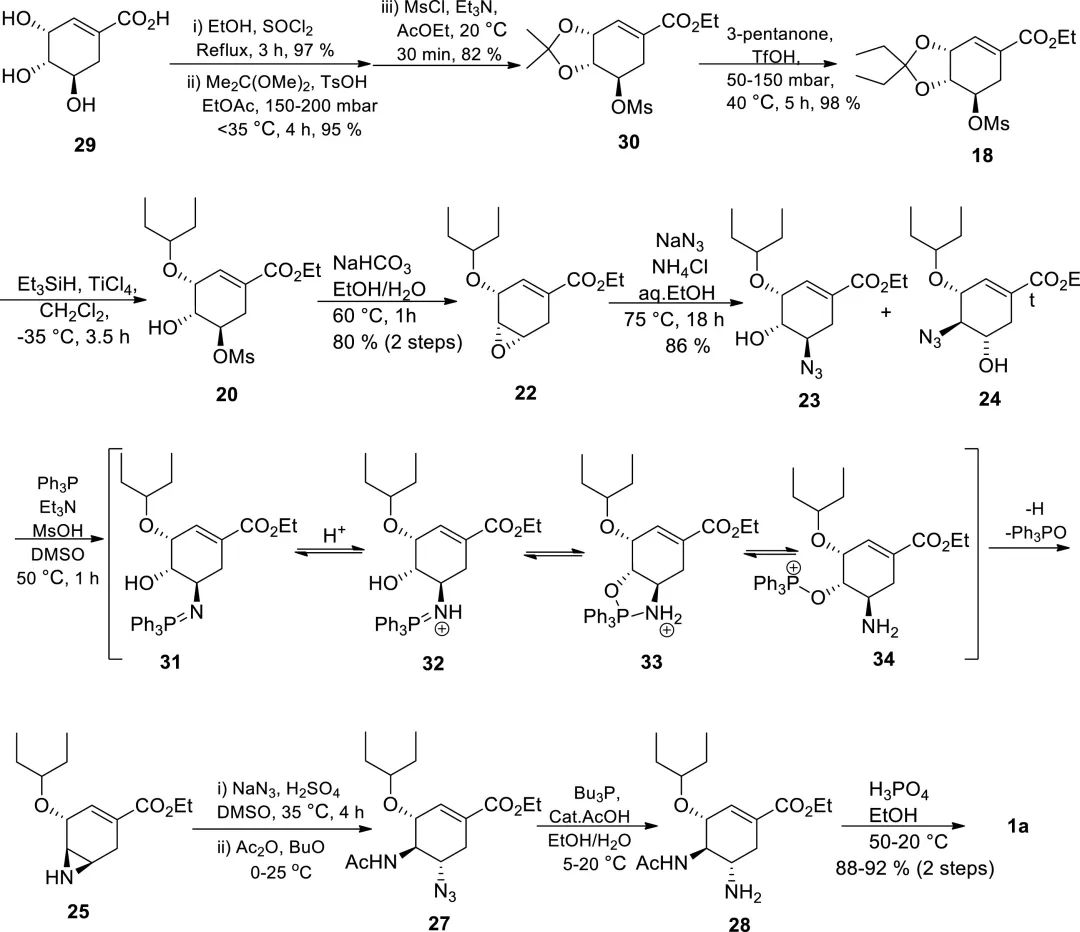
Figure 4. Roche’s 12-step industrial synthesis route.
Currently, the only industrial synthesis route for Tamiflu is still the Roche route, but this route has three potential problems: a) it requires shikimic acid, a natural product isolated from the Chinese star anise plant, whose purity and quantity have always been unstable; b) large-scale safe handling of potentially explosive azide reagents and intermediates; c) the synthesis route is long and the overall yield is low. These shortcomings have prompted researchers to develop truly efficient, safe, cost-effective and environmentally friendly alternative routes, and more than 70 synthetic routes have been reported so far.4. Alternative synthetic methods that rely on shikimic acid
After the availability of (−)-shikimic acid increased over time, researchers set out to develop shorter and more productive synthetic routes. These routes can be divided into two groups: azide chemistry-dependent routes and azide-free routes.4.1 Azide-dependent route (3 representative routes)
The Shi group developed an optimized 8-step route with a total yield of 47%. The key improvement is the regio- and stereo-selective nucleophilic substitution of OMs at the allylic position to obtain azide intermediate 37, which is then used in the presence of triethylamine and a large amount of water (Staudinger reaction) to obtain aziridine 41. After acetylation, 3-pentanol reacts with aziridine 41 under Lewis acid catalysis to selectively open the ring to give compound 40. Finally, it is treated with sodium azide to obtain Tamiflu.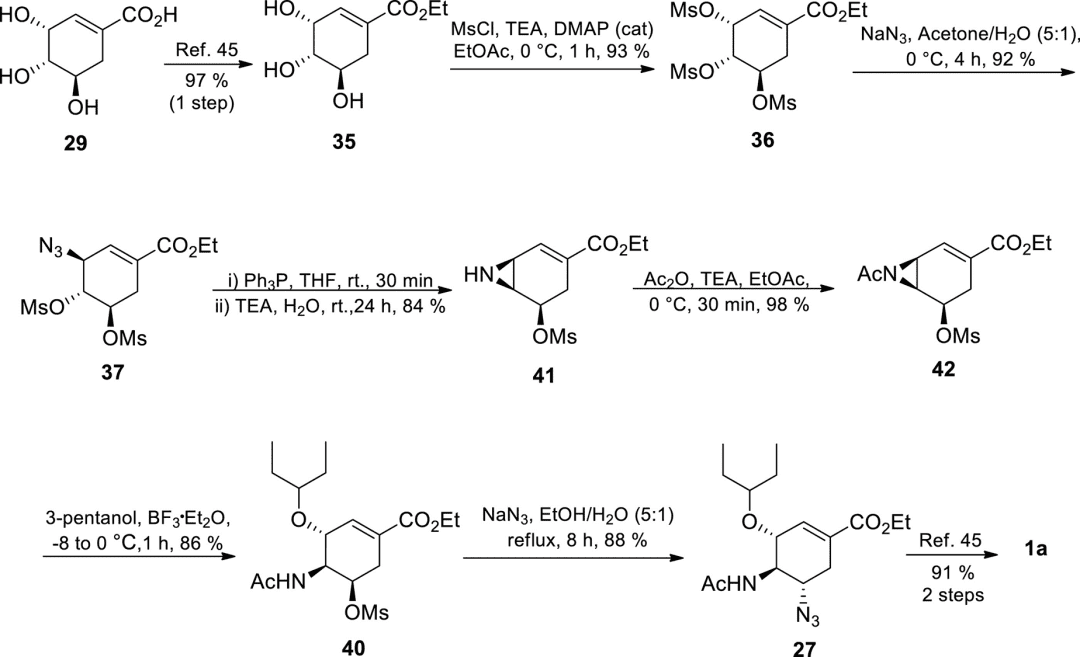
Figure 5. The 8-step route developed by the Shi group.
Subsequently, the Shi group reported an improved 11-step synthetic route based on previous work, with a total yield of 55%. Starting from shikimic acid, Tamiflu was obtained through the 3,4-cyclic sulfite intermediate 43. The yield of each step in this route exceeded 90%, and the subsequent steps could be carried out without purification of the crude product.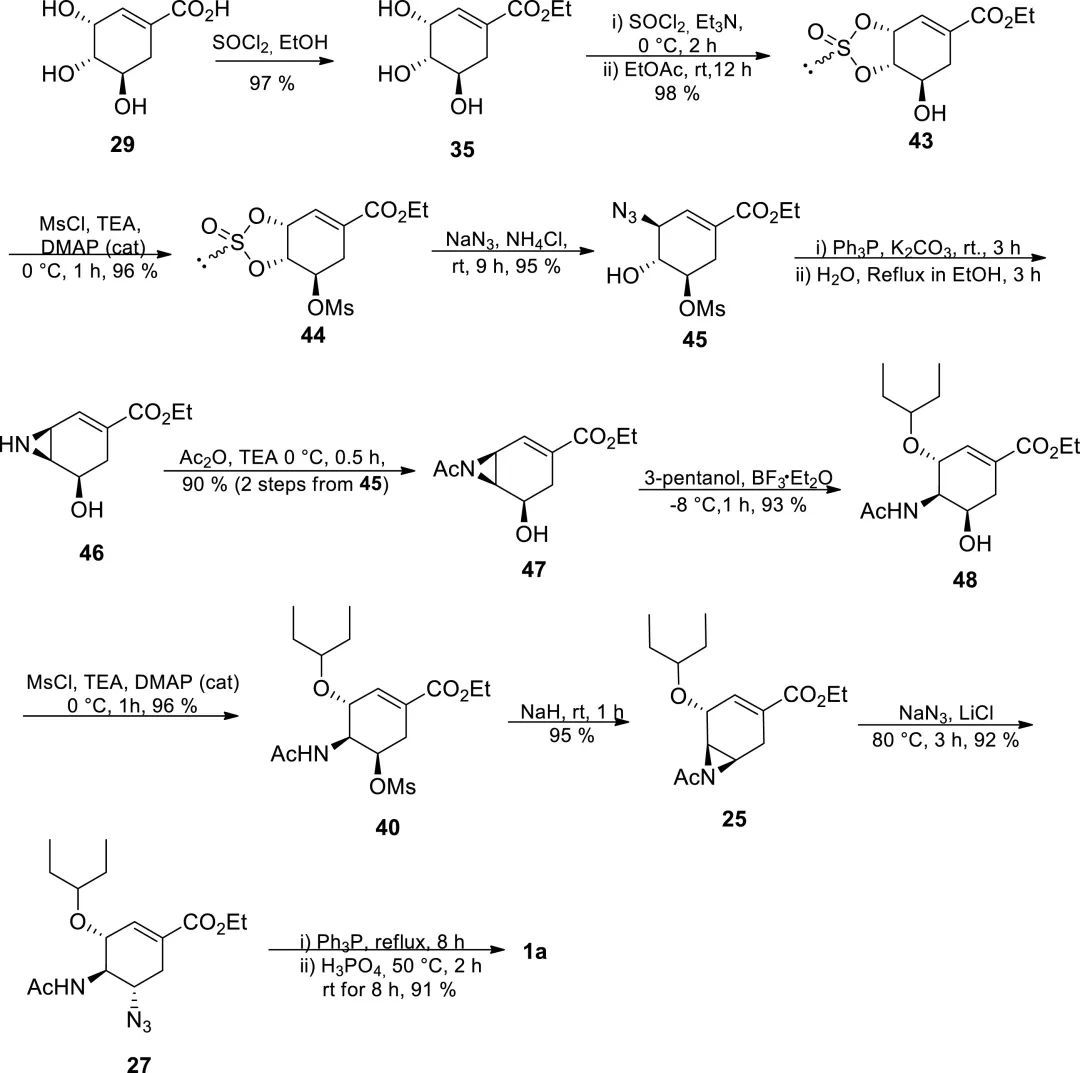
Figure 6. Improved 11-step route developed by Shi group.
The Watts group reported an 8-step all-flow chemical synthesis starting from ethyl shikimate 35. Flow chemistry techniques have the advantages of high efficiency, easy scale-up, safety and reproducibility, allowing the generation and consumption of hazardous intermediates in situ, preventing their accumulation, thereby improving process safety. In addition, microreactors can handle exothermic heat dissipation very well due to the inherent high surface area to volume ratio and fast reaction. Industry uses this technique to produce up to 2000 tons of chemicals per year. The technique first treated 36 with MsCl in the presence of TEA at room temperature and ultrasound treatment, followed by treatment with sodium azide to give the azide 37. Treatment with (EtO)3P at 190 °C gave the aziridine 38b, which was then ring-opened with 3-pentanol at 100 °C to give 39. Subsequent N-P bond cleavage of 39 using sulfuric acid at 170 °C formed intermediate 49 in situ, which was subsequently acetylated at room temperature to give acetamide 40. It was then treated with sodium azide to give azide 27, which was then sonicated with NaBH4 in the presence of CoCl2 at room temperature to give oseltamivir. The total yield was 58% with a total residence time of 3.5 min, compared to more than 30 h for conventional synthesis.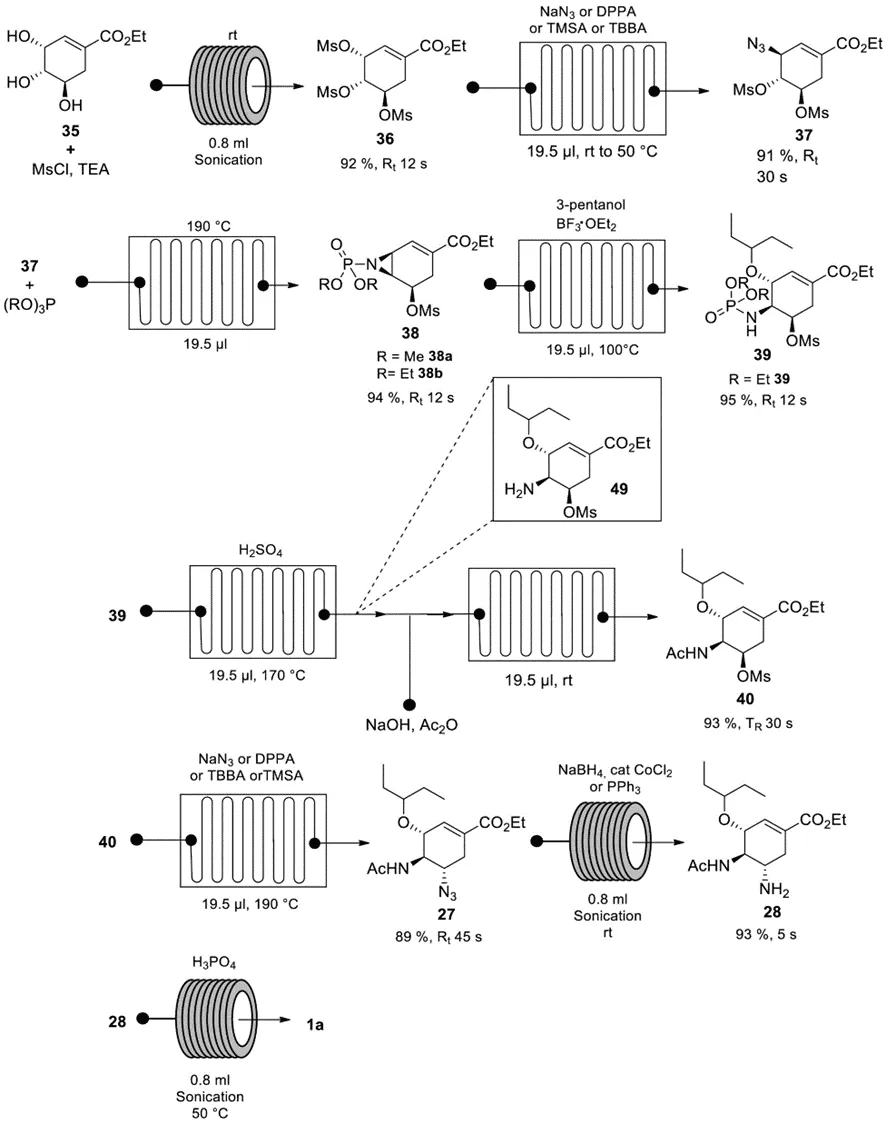
Figure 7. Continuous flow synthesis route by Watts et al.
4.2 Shikimic acid-dependent azide-free route
In 2000, Roche reported an azide-free conversion of epoxide 22 to tamiflu using tBuNH2 as the nitrogen nucleophile with an overall yield of 35–38%, comparable to the industrial route.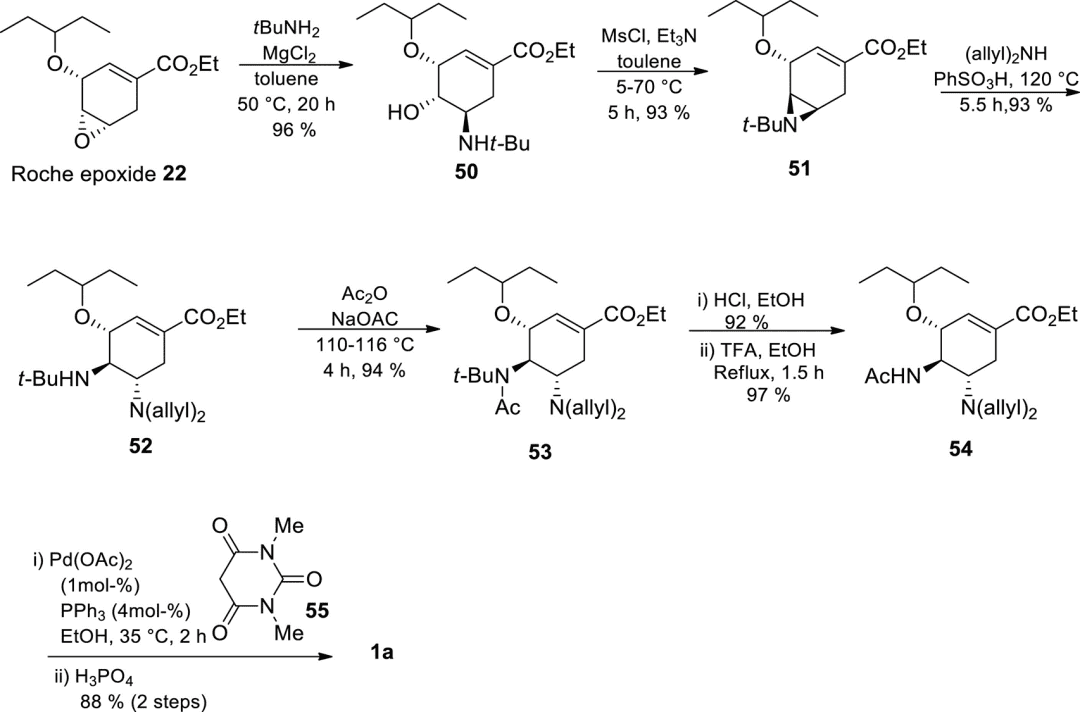
Figure 8. Roche’s azide-free route starting from epoxide 22.
In 2013, Shi group shortened the strategy from 9 steps to 6 steps, while increasing the yield from 35-38% to 61-69%.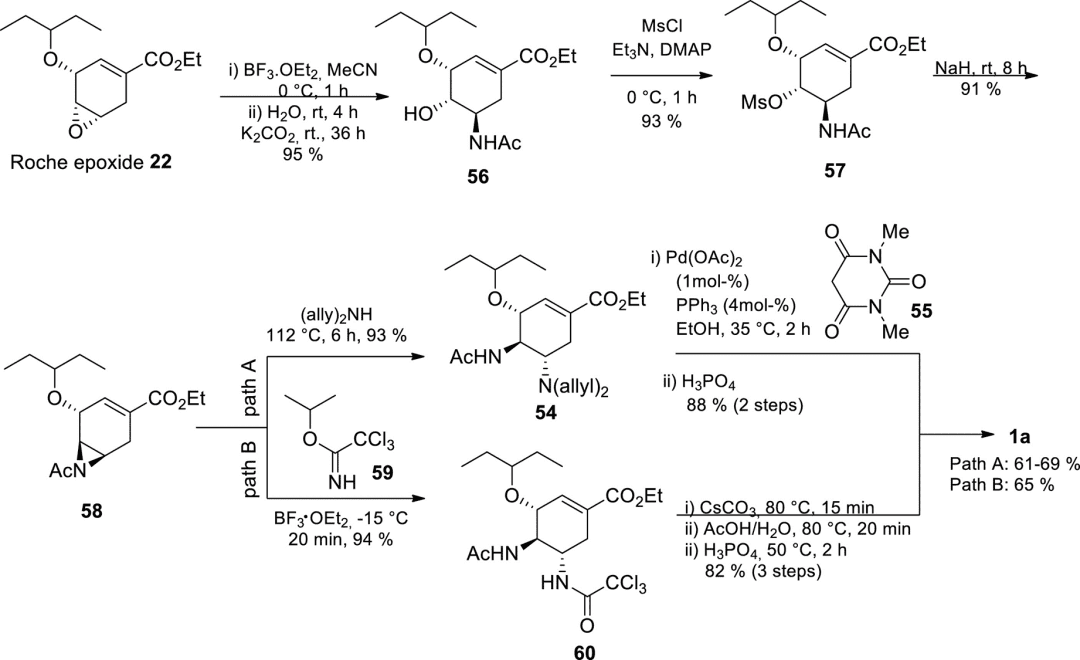
Figure 9. Azide-free route from epoxide 22 by the Shi group.
Alternative synthetic methods that do not rely on shikimic acid
Synthesis via DA reaction
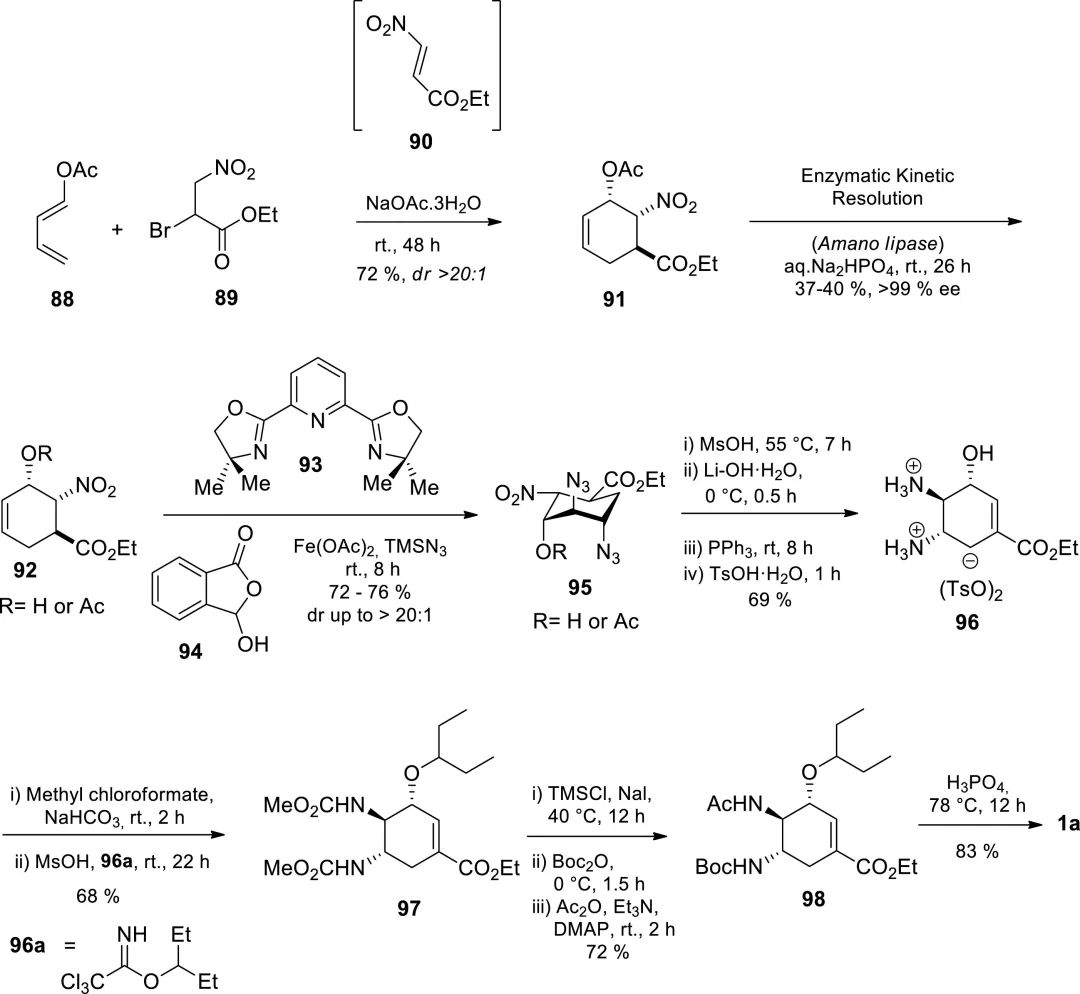
Figure 10. Enantioselective synthesis of Tamiflu via iron-catalyzed stereoselective olefin diazidation.
5.2 Through HWE reaction/aldol condensation/Michael addition
First, 118 was obtained through Michael addition, and then 121 was obtained through tandem HWE and Michael addition. Then, 113 was obtained through Michael addition with 4-methylthiophenol (113 and 114 should not have olefins, which is probably a typo by the author). After reducing the nitro group, 4-methylthiophenol was eliminated with a base to obtain the double bond again. The total yield of this route is 46%, and only two intermediate separation operations are required.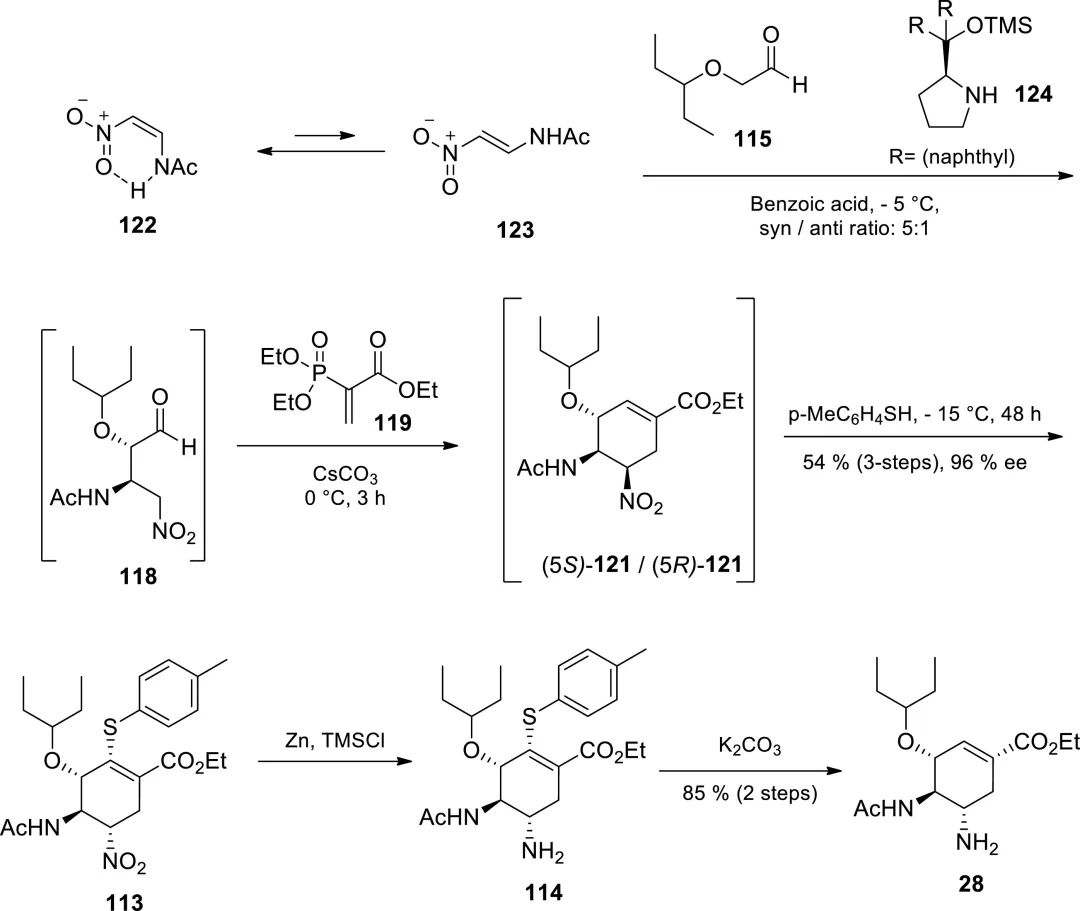
Figure 11. Ma group synthesized Tamiflu via Michael addition and HWE reaction.
5.3 Synthesis from sugars
Cheap and abundant sugars such as D-xylose, D-ribose, D-mannitol, and D-glucose have also been used as starting materials to synthesize Tamiflu. However, the synthetic process relying on sugars is characterized by long duration (12-22 steps), frequent chromatographic purification (9-16 times), and low yield (2.6-15%).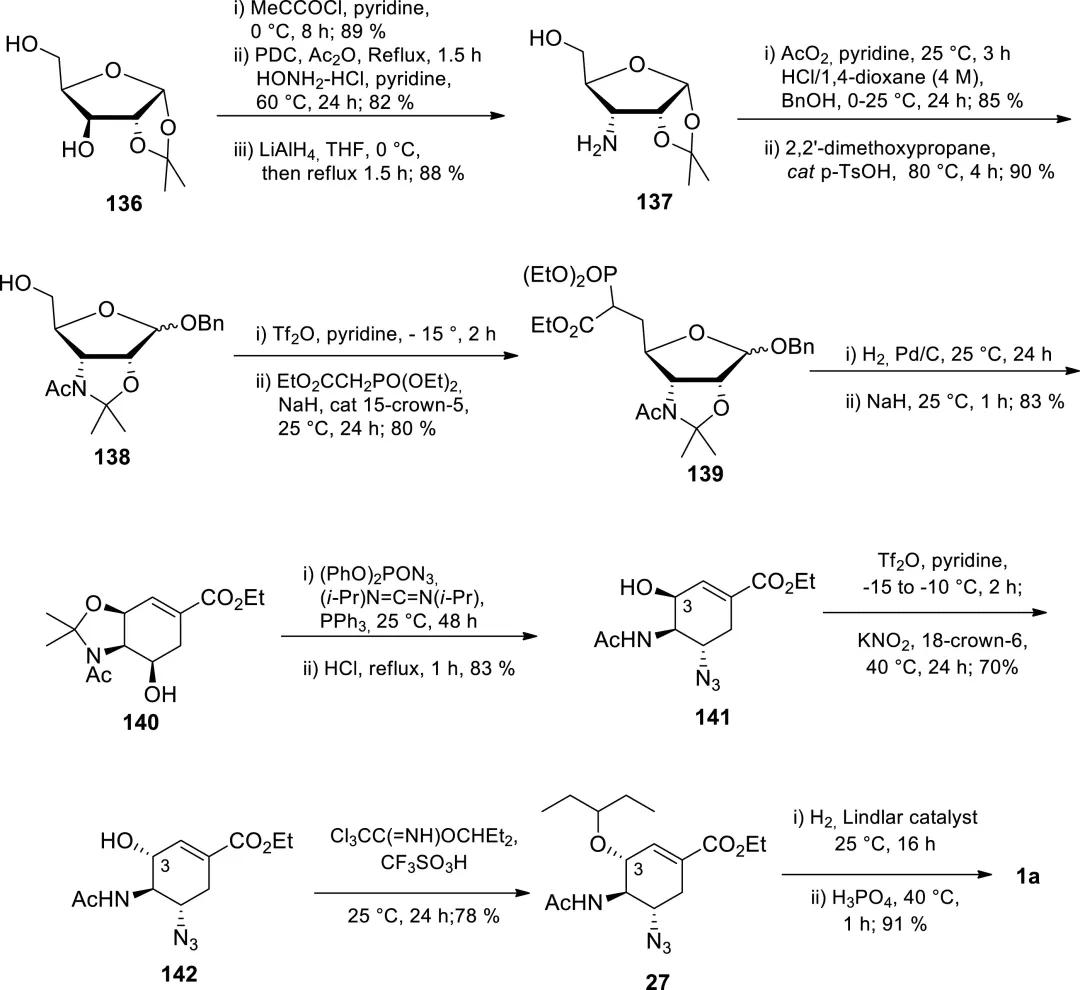
Figure 12. Wong et al.’s enantioselective synthesis of Tamiflu from D-xylose.
6. Summary
1) Compared with existing industrial routes, most of the synthetic routes without shikimic acid and azide have lower yields, but there are a few routes with yields exceeding the industrial routes, but they have not yet been industrialized. 2) The availability of shikimic acid has been solved by developing more efficient extraction and purification processes or using genetically engineered Escherichia coli fermentation. 3) The application of continuous flow technology in the synthesis of Tamiflu is a potential tool for safely handling hazardous azide compounds and improving efficiency.Reference
- Sagandira, C. R., Mathe, F. M., Guyo, U., & Watts, P. (2020). The evolution of Tamiflu synthesis, 20 years on: Advent of enabling technologies the last piece of the puzzle. Tetrahedron, 76(37), 131440. DOI