The Promising World of Target Drug Exploration
Abstract
While target-based drug discovery is the prevailing method in drug development, recent comprehensive assessments indicate its results are subpar. The approach yields a relatively small number of drugs, with therapeutic effects often dependent on off-target mechanisms. This raises critical questions: Is this approach as effective as believed, or is it merely superficially attractive but lacking in substantial value? Drawing on the research of Arash Sadri, this article will delve into the efficacy of target-based drug discovery and its interaction with non-target mechanisms.
Challenges and dilemmas behind drug discovery
A review of literature spanning 150 years reveals that only around 9.4% of small molecule drugs were discovered through target-based methods. While the ratio of approved drug candidates to those entering clinical studies is approximately 13%, it drops significantly to 0.4% in complex fields like central nervous system diseases and cancer. Furthermore, drug research and development face numerous challenges, including low efficiency, high costs, and pharmaceutical company withdrawals.
Researchers have highlighted that current mainstream target-based drug discovery methods exhibit clear reductionist tendencies. This approach focuses on identifying drugs that can manipulate a single or a few proteins while overlooking the intricate network structures and feedback mechanisms within and outside cells. Despite its longstanding popularity, this approach has coincided with diminishing productivity in drug development. Therefore, there is a pressing need to reassess and enhance existing drug development strategies to address present challenges.
Reflection on the current state and future challenges of targeted drug research and development
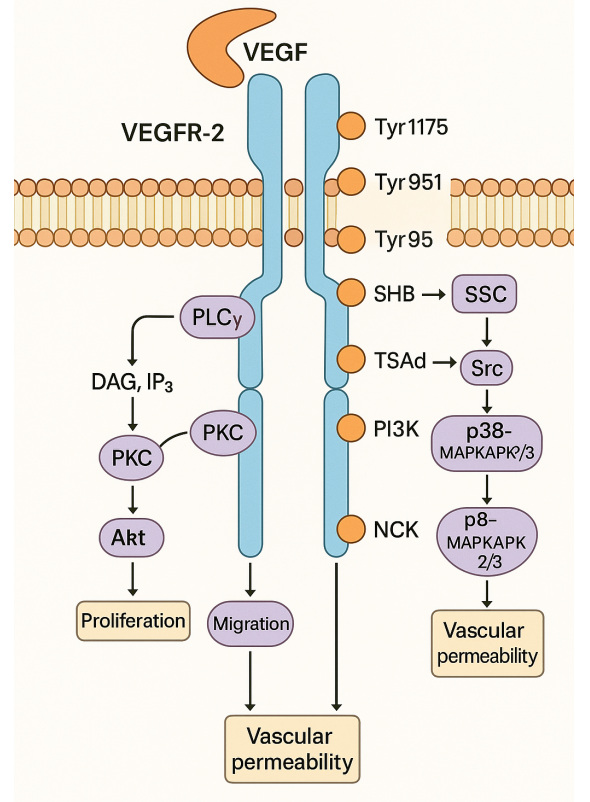
The efficiency and effectiveness of target-based drug discovery methods have long been a subject of debate. Researchers have proposed a hypothesis suggesting that the decline in drug discovery efficiency may be linked to an excessive reliance on target-based reductionist approaches. This method primarily focuses on the interaction of drugs with a limited number of predetermined “target” proteins, shaping molecule selection and optimization, often considering in vivo and human data only at later stages.
To investigate this hypothesis, researchers manually analyzed all drugs approved by the US FDA up to the end of 2020, focusing on the origins of drug discovery. To ensure objectivity and accuracy, they defined key terms:
“Origin of discovery”: when a correlation between a treatment category and effect is first observed.
“Therapeutic class”: chemically or pharmacologically similar substances to a lead molecule.
“Target-based drugs”: discovery relies mainly on observing molecule effects on proteins.
“Phenotype-based drugs”: discovery relies mainly on observing molecule effects on organism phenotype.
Their analysis revealed that traditional methods, unlike target-based ones, are more empirical. They select and optimize molecules based on their therapeutic effects on humans and other organisms, lacking tools to evaluate effects on a single protein. To draw comprehensive conclusions, researchers expanded the study to cover all approved drugs, enhancing analysis accuracy and objectivity.
Exploring new avenues for phenotypic drug discovery: moving beyond “targeted” mechanisms to embrace “non-targeted” alternatives
In stark contrast to the prevalent approach of targeting specific molecules, an emerging strategy focuses on “non-targeted” mechanisms, emphasizing the intricate interactions between drugs and multiple proteins. While target-based reductionist methods dominate current drug discovery, this evidence-driven, anti-reductionist approach holds promise for enhancing overall efficiency. Leveraging advanced tools like artificial intelligence and machine learning, this approach prioritizes higher-level phenotypic observations, which more closely mirror patients’ actual responses to treatment. By considering the various mechanisms of drug action comprehensively and avoiding excessive fixation on a single target, this method offers a more holistic strategy for drug development.
Research data indicates that a significant proportion of approved drugs are phenotype-based. The discovery of these drugs primarily stemmed from observations of non-human or in vitro phenotypes, human phenotypes, phenotypic effects of endogenous molecules, and historical use of compounds and their mechanisms of action. This underscores the indispensable and pivotal role of phenotype-based drug discovery in drug development, extending beyond the modulation of single proteins.
Comparing the proportions of phenotype-based and target-based drugs among all approved drugs and those approved after 1995 reveals an intriguing trend: 1995 marked a significant turning point with the approval of saquinavir (S126514), the first “target-based” drug. This event delineates the evolution of these two methods, highlighting the growing importance of phenotype-based approaches in modern drug discovery.
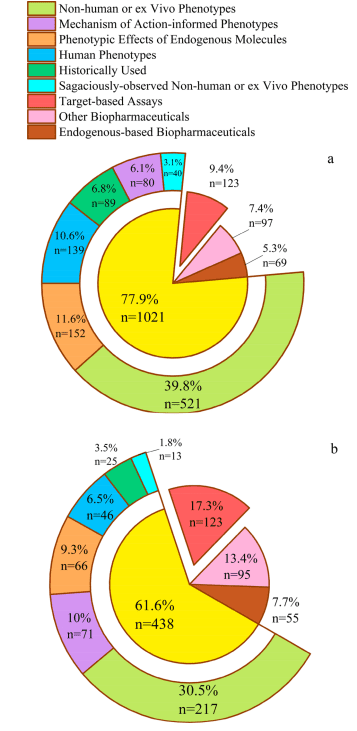
Figure 1. Proportion of different methods in the origin of drug discovery. (a) All approved drugs; (b) Drugs approved after 1995.
While the proportion of targeted drug research and development (R&D) in approved drugs has shown a consistent upward trend over the years, it has never exceeded the proportion of phenotypic drug R&D. Interestingly, many drugs labeled as “targeted” actually exhibit a range of “non-targeted” therapeutic mechanisms. For instance, donepezil (D332795), initially developed as an acetylcholinesterase inhibitor, has been found through subsequent research to possess up to 40 independent therapeutic mechanisms, including anti-inflammatory and neuroprotective effects, among others.
Furthermore, we have observed that many approved drugs do not necessarily bind strongly to their therapeutic “target,” although they tend to rank in the high-affinity percentile. This suggests a correlation between high-affinity binding to a “target” and therapeutic efficacy. However, it’s crucial to note that high-affinity binding to a single “therapeutic target” is just one of many factors influencing a drug’s effectiveness in treatment.
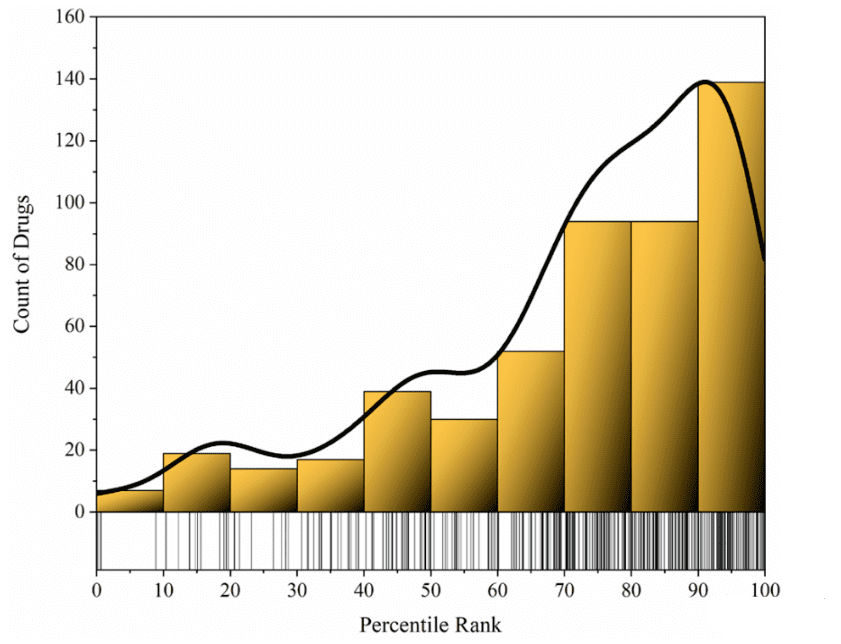
Figure 2. Affinity percentile ranks of all ChEMBL ligands for approved drugs relative to their therapeutic targets
Overall, target-based approaches to drug discovery may oversimplify the complexity of biological systems. In contrast, high-level phenotypic observation of drugs offers a more efficient way to study and treat disease by directly targeting treatment effects, which tends to be more predictive. This approach treats the complexity of the human body as a “black box” and does not delve into low-level mechanisms but focuses directly on the ultimate therapeutic effect.
Crucial target-based drug discovery remains vital and indispensable
Target-based drug discovery holds a crucial role in the extensive process of drug research and development. Early on, technologies like computational chemistry, high-throughput screening, and combinatorial chemistry garnered significant attention and favor from the research and development (R&D) community. However, as scientific and technological advancements have progressed and research has deepened, the limitations of these technologies have gradually become apparent. Despite this, these technologies remain indispensable core elements in the current drug development process.
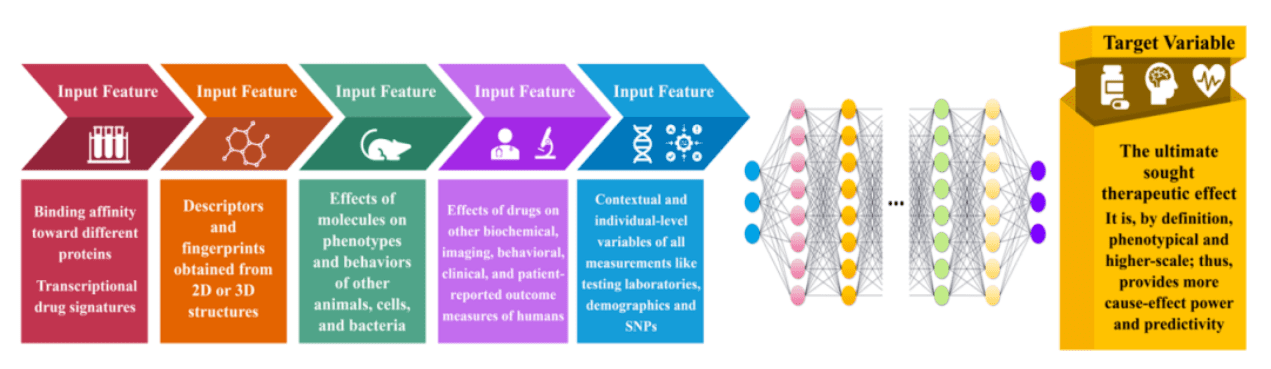
In diseases like single-gene Mendelian disorders, target-based drug development methods have proven their unique value, as seen in successful monoclonal antibody development. Target-based strategies are also key in finding analogs and enhancing drug structures. Yet, collecting advanced data for complex diseases can be costly and challenging. Here, target-based drug development offers a strong foundation for R&D efforts.
However, strategies focused solely on a single target face clear limitations. To address this, researchers are delving into polypharmacology, aiming to enhance drug efficacy by targeting multiple sites simultaneously. Additionally, novel approaches like systems pharmacology and network pharmacology offer holistic perspectives, providing comprehensive solutions for complex diseases by addressing multiple underlying factors.
While target-based drug development has clear advantages, particularly in diseases closely linked to specific proteins, future approaches must consider the broader complexity of diseases. To enhance the efficiency and effectiveness of drug R&D, we must integrate evidence-based research and leverage new technologies to delve deeply into disease mechanisms. Only through this comprehensive approach can we deliver more innovative solutions to drug R&D.
Summary
Target-based drug discovery continues to be fundamental in drug R&D. Future trends will emphasize integrating various technologies and methods to address drug R&D challenges comprehensively and predictively. With advancements in science and technology, a deeper understanding of diseases is needed to meet public health needs and industry challenges. This transformation will drive ongoing progress in drug R&D, contributing significantly to human health.