Targeting β-Adrenergic Receptor Signaling: A Promising Strategy in Cancer Therapy
Abstract
The nervous system plays a crucial role in cancer progression by influencing both the tumor microenvironment (TME) and macroenvironment through neural and humoral pathways. β-Adrenergic receptors (β-ARs), activated by neurotransmitters such as norepinephrine and epinephrine, contribute to key cancer processes including cell proliferation, angiogenesis, immune evasion, and metastasis. Recent research has highlighted the importance of β-AR signaling in tumor progression, with β-adrenergic activation influencing tumor growth, immune suppression, and metastasis through interactions with various tissues and organs in the host. Additionally, psychosocial factors, particularly chronic psychological stress, have been shown to activate the sympathetic nervous system, further enhancing tumor dynamics. The growing understanding of β-adrenergic signaling presents new therapeutic possibilities, particularly through β-blockers, which have shown potential in inhibiting tumor growth, metastasis, and enhancing the efficacy of other cancer treatments. This review explores the complex interplay between β-adrenergic receptor signaling, cancer progression, and the broader implications for treatment and prevention.
Introduction: The Complex Interplay Between Cancer and the Nervous System
Processes related to the transformation of normal cells to cancerous ones and those related to the proliferation of cancer cells and development of metastases are tightly modulated by both tumor micro- and macroenvironments. Whereas the tumor microenvironment represents a milieu in which transformation occurs and cancer cells interact with cells of the surrounding tissue and tissue structures, the macroenvironment is essential, especially for the systemic immune response of the host to cancer and nourishment of cancer tissue. For centuries, it was suggested that somatic and psychosocial factors may significantly affect cancer development and progression. However, it is only recently that the neuroendocrine–immune pathways and mechanisms interconnecting psychosocial, somatic factors, and cancer have been elucidated in more detail. The sympathoadrenal system plays a crucial role in these pathways and mechanisms.
The interaction between the nervous system and cancer is particularly complex, as it involves both neural pathways (such as the autonomic nervous system) and humoral pathways (hormones and neurotransmitters). One key area of focus is the role of the sympathoadrenal system, which is responsible for releasing neurotransmitters like norepinephrine and epinephrine. These catecholamines exert significant influence on tumor biology through their action on β-adrenergic receptors located in various tissues, including those within tumors.
Over the past two decades, a growing body of evidence has highlighted the importance of β-adrenergic receptor signaling in the cancer process. These receptors, particularly the β1, β2, and β3 subtypes, are found not only in tumor cells but also in surrounding stromal cells, immune cells, and endothelial cells. β-Adrenergic signaling contributes to tumor progression by promoting cellular proliferation, angiogenesis, and immune evasion. Additionally, these receptors are implicated in the metastasis of tumors to distant sites by enhancing the migratory capabilities of cancer cells.
Moreover, emerging research suggests that the nervous system’s influence on cancer is not limited to physiological processes alone but extends to psychosocial and spiritual factors. Psychological stress, for example, has been shown to activate the sympathetic nervous system, thereby further influencing tumor dynamics. In this context, β-adrenergic signaling acts as a bridge between the mind, body, and tumor environment.
This article explores the intricate role of β-adrenergic receptor signaling in cancer and its broader implications for both treatment and prevention.
Understanding β-Adrenergic Receptor Signaling in Cancer
β-Adrenergic receptors (β-ARs) are a group of G protein-coupled receptors (GPCRs) that play a central role in mediating the effects of catecholamines, including norepinephrine and epinephrine. These receptors are classified into three main subtypes: β1, β2, and β3, each with distinct tissue distribution and functional roles. In the context of cancer, β-adrenergic receptor signaling has emerged as a key modulator of tumor biology, influencing processes such as cell proliferation, angiogenesis, immune evasion, and metastasis.
The activation of β-ARs by norepinephrine and epinephrine triggers intracellular signaling cascades that regulate a variety of cellular processes. Upon binding to β-ARs, these catecholamines activate adenylate cyclase, increasing intracellular cyclic AMP (cAMP) levels. Elevated cAMP activates protein kinase A (PKA), which in turn regulates a host of downstream targets, including transcription factors, ion channels, and enzymes that govern tumor progression.
Research has shown that β-AR signaling contributes to cancer progression by promoting tumor cell proliferation and survival. In particular, β2-AR activation has been associated with enhanced tumor growth and resistance to apoptosis. Furthermore, β-AR signaling also supports the process of angiogenesis, which is critical for supplying growing tumors with oxygen and nutrients. The stimulation of β-ARs has been found to increase the expression of vascular endothelial growth factor (VEGF), a potent angiogenic factor, thereby aiding in tumor vascularization.
Beyond the direct effects on tumor cells, β-AR signaling also influences the tumor microenvironment (TME). Studies have shown that β-adrenergic activation can modulate immune cell function, contributing to immune evasion and promoting an immunosuppressive TME. Additionally, β-AR signaling enhances the motility and invasiveness of cancer cells, facilitating metastasis to distant organs.
Overall, β-adrenergic receptor signaling represents a critical pathway in cancer progression, offering potential targets for therapeutic intervention aimed at halting tumor growth and metastasis.
The Impact of β-Adrenergic Signaling on Cancer Progression
Extensive in vitro and in vivo research has documented the significant effects of epinephrine (EPI) and norepinephrine (NE) on cancer. Studies have consistently demonstrated that exposure to NE or EPI leads to increased cancer cell proliferation in cell cultures and enhances cancer incidence and progression in animal models subjected to stressors. Conversely, strategies that mitigate the sympathoadrenal system’s influence—such as chemically destroying sympathetic nerve endings, removing the adrenal medulla, or using synthetic antagonists to block adrenergic receptors have been shown to inhibit cancer development and growth.
Recent advancements have shed light on the cellular and molecular mechanisms through which NE and EPI stimulate cancer. These catecholamines influence tumorigenesis, cancer cell proliferation, and metastasis via multiple downstream pathways within both the tumor microenvironment and macroenvironment. This is facilitated by the widespread presence of β-adrenergic receptors (β-ARs) not only within the tumor microenvironment but also across various cell types throughout the body. NE and EPI, primarily released from sympathetic nerve endings and the adrenal medulla, activate these receptors. Locally, NE released from sympathetic nerve fibers densely innervating the tumor area directly stimulates β-ARs on both tumor and stromal cells. Systemically, circulating EPI and NE, along with NE from sympathetic nerves in other organs like lymphatic tissues, indirectly influence cancer biology.
β-Adrenergic Signaling in the Tumor Microenvironment
Within the tumor microenvironment, NE and EPI enhance several cellular and molecular processes that drive cancer initiation and progression. Elevated levels of these catecholamines often correlate with more aggressive tumor behavior. β-adrenergic signaling impacts nearly all of the cancer hallmarks defined by Hanahan and Weinberg, including sustained proliferative signaling, evasion of apoptosis, and angiogenesis.
Interestingly, tumors may not only receive NE and EPI from external sources but also synthesize these catecholamines internally. Sympathetic nerve fibers innervating tumors can locally release NE, while neuronal progenitor cells from the central nervous system may infiltrate tumors and adopt an adrenergic phenotype, producing NE and EPI directly within the tumor microenvironment. This local production complicates the catecholaminergic influence on tumors, suggesting a more intricate interaction than previously understood.
Molecular Mechanisms of β-Adrenergic Influence
β-adrenergic signaling induces genome instability by increasing DNA damage and impairing repair mechanisms. Studies have shown that NE and EPI exposure leads to double-strand DNA breaks in cancer cells, an effect that can be mitigated by β-blockers like propranolol. Additionally, β-AR activation promotes proliferative signaling pathways, such as PI3K/Akt and ERK1/2, enhancing tumor cell growth and survival. However, the role of β-ARs can vary depending on the cancer type and receptor subtype involved.
Moreover, β-adrenergic signaling contributes to cancer cell resistance to apoptosis. Blocking β2-adrenergic receptors has been shown to induce cell cycle arrest and apoptosis in various cancer models, highlighting the therapeutic potential of β-blockers in reducing tumor viability [58,59,60]. Additionally, β-AR activation facilitates cancer cell motility, invasion, and metastasis, although findings are sometimes conflicting, indicating a complex role of β-ARs in different cancer contexts.
Therapeutic Potential of Targeting β-Adrenergic Signaling
Given the multifaceted role of β-adrenergic signaling in cancer progression, targeting this pathway presents a promising therapeutic strategy. β-blockers, which inhibit β-ARs, have demonstrated efficacy in reducing tumor growth, metastasis, and enhancing the effectiveness of conventional cancer treatments. Ongoing research aims to further elucidate the mechanisms by which β-AR antagonists can be integrated into cancer therapy to improve patient outcomes.
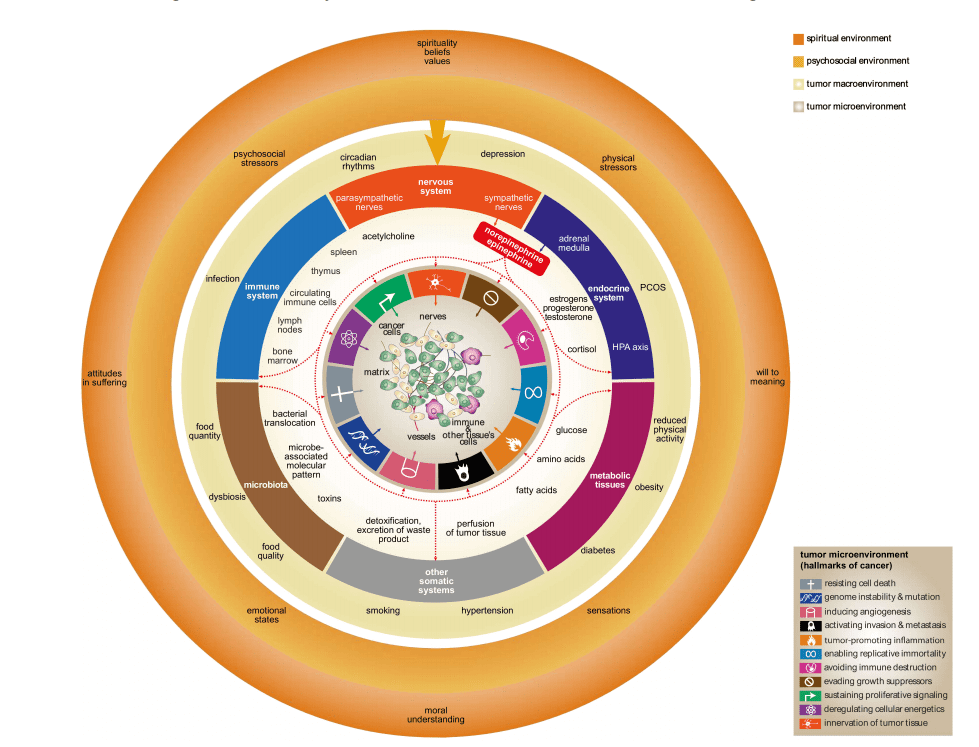
Figure 1. β-adrenergic signaling mediates the effect of the brain on the tumor micro- and Macroenvironments.
Psychosocial and Spiritual Factors: The Unseen Role in Cancer Progression
In recent years, there has been growing recognition of the profound impact that psychosocial and spiritual factors can have on cancer progression. While the role of genetics, environment, and lifestyle in cancer development is well-documented, the influence of the mind-body connection, particularly through stress and emotional well-being, has become an area of increasing interest in cancer research. Psychological stress, for example, has been shown to activate the sympathetic nervous system (SNS), leading to the release of catecholamines such as norepinephrine and epinephrine. These neurotransmitters, in turn, engage β-adrenergic receptors (β-ARs) on tumor cells and stromal components, modulating tumor biology and influencing cancer progression.
The link between stress and cancer has been established through both animal models and human studies. Stressful experiences, particularly chronic psychological stress, have been associated with increased tumor growth, metastasis, and resistance to treatment. This is primarily mediated through β-AR signaling pathways, which promote processes like angiogenesis, immune suppression, and tumor cell migration. For instance, β-adrenergic activation can enhance the expression of vascular endothelial growth factor (VEGF), driving angiogenesis and providing tumors with the blood supply they need for continued growth. Additionally, stress-induced activation of β-ARs in immune cells can lead to the suppression of anti-tumor immunity, favoring tumor evasion from immune surveillance.
Spirituality, too, plays a significant role in shaping cancer outcomes, albeit in a more indirect manner. Spiritual well-being, coping mechanisms, and a sense of purpose have been shown to positively influence emotional resilience, reduce stress, and improve overall survival in cancer patients. It is believed that the psychological benefits of spiritual practices may help to counteract the negative effects of chronic stress on the body’s biological systems, including the sympathetic nervous system.
Thus, both psychosocial stress and spiritual factors are integral components of the cancer journey, influencing tumor biology through complex interactions with β-adrenergic receptor signaling.
Therapeutic Implications: Targeting β-Adrenergic Receptor Signaling in Cancer Treatment
The growing understanding of β-adrenergic receptor (β-AR) signaling in cancer has opened new avenues for therapeutic intervention. Since β-adrenergic receptors are involved in key processes like tumor growth, metastasis, immune modulation, and angiogenesis, targeting these receptors could represent a promising strategy to limit cancer progression and improve treatment outcomes. The potential of β-blockers—drugs that inhibit β-AR signaling—has been a key focus of research in this area, especially given their widespread use in managing cardiovascular diseases.
β-blockers, such as propranolol, have shown promising effects in preclinical and clinical studies for a variety of cancers, including breast, prostate, and ovarian cancer. By blocking the β-ARs, these drugs can attenuate the pro-tumorigenic effects of catecholamines, including the suppression of immune responses, promotion of angiogenesis, and enhancement of cell survival. Studies have demonstrated that β-blocker treatment can reduce tumor growth, inhibit metastasis, and enhance the efficacy of chemotherapeutic agents. For example, in breast cancer, propranolol has been found to inhibit the growth and migration of cancer cells, both in vitro and in vivo.
In addition to β-blockers, there is growing interest in developing novel drugs that specifically target β-AR signaling pathways involved in cancer progression. For instance, selective β2-AR antagonists have been explored as potential cancer therapeutics due to their ability to disrupt the β2-mediated pathways that drive tumor metastasis and immune suppression. Furthermore, β-AR signaling inhibitors, in combination with traditional cancer treatments like chemotherapy or immunotherapy, could synergistically improve therapeutic outcomes by enhancing immune surveillance and reducing tumor growth.
Despite promising results, more clinical trials are needed to better understand the full therapeutic potential of β-AR targeting in cancer treatment. Ongoing research aims to identify biomarkers for selecting patients who are most likely to benefit from β-AR-based therapies and optimize their integration into cancer treatment regimens.
References
- Antoni, M. H., Lutgendorf, S. K., Cole, S. W., Dhabhar, F. S., Sephton, S. E., & McDonald, P. G. (2006). The influence of bio-behavioral factors on tumor biology: pathways and mechanisms. Nature Reviews Cancer, 6(3), 240–248. DOI
- Fidler, I. J. (2003). The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nature Reviews Cancer, 3(6), 453–458. DOI
- Hwang, J., & Lee, J. H. (2016). The role of the β-adrenergic receptor in cancer. Journal of Cancer Research and Clinical Oncology, 142(3), 529–538. DOI
- Lee, K. Y., & Lee, D. H. (2018). Sympathetic nervous system regulation of cancer metastasis. Cancer Research, 78(10), 2489–2494. DOI
- McEwen, B. S. (2007). Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews, 87(3), 873–904. DOI
- Powe, D. G., Vasselli, J. R., & Sood, A. K. (2010). The sympathetic nervous system in cancer. Cancer Research, 70(24), 10850–10858. DOI
- Siegel, R. L., Miller, K. D., & Jemal, A. (2019). Cancer statistics, 2019. CA: A Cancer Journal for Clinicians, 69(1), 7–34. DOI
- Zhang, J., & Wang, Z. (2017). The role of the sympathetic nervous system in cancer: Therapeutic implications. Journal of Oncology, 2017, 1–11. DOI
- Yang, L., & Zhang, Y. (2018). β-Adrenergic receptors and cancer: A new approach to cancer therapy. Cancer Treatment Reviews, 69, 77–83. DOI
- Sood, A. K., & Fletcher, M. S. (2011). β-adrenergic signaling in the tumor microenvironment. Clinical Cancer Research, 17(24), 7382–7390. DOI
- Liu, J., & Gao, H. (2014). β-adrenergic signaling in cancer: A potential new target for cancer therapy. Cancer Research, 74(6), 1613–1621. DOI
- Zhou, J., & Ma, Q. (2017). The impact of β-adrenergic signaling on the tumor microenvironment. Journal of Cancer Research and Clinical Oncology, 143(4), 633–640. DOI
- Wirtz, D., & Sweeney, L. (2014). β-adrenergic signaling in cancer: Role in tumor microenvironment and progression. Cancer Research, 74(3), 818–827. DOI
- Alvarado, D., & Thomas, S. (2016). The tumor microenvironment and β-adrenergic receptor signaling in metastasis. Clinical Cancer Research, 22(7), 1670–1680. DOI
- Mravec, B., Horvathova, L., & Hunakova, L. (2020). Neurobiology of cancer: The role of β-adrenergic receptor signaling in various tumor environments. International Journal of Molecular Sciences, 21(20), 7958. DOI




