Targeting DLL3 in Small Cell Lung Cancer: New Hope for Treatment Breakthroughs
Abstract
Small cell lung cancer (SCLC) remains one of the most aggressive and challenging cancers to treat, with poor survival rates and limited therapeutic options for advanced stages. Recent advances have identified Delta-like ligand 3 (DLL3) as a promising target for SCLC therapy due to its selective expression in cancerous tissues and minimal presence in normal cells. This paper reviews the potential of DLL3-targeted therapies, including antibody-drug conjugates, bispecific T-cell engagers, and chimeric antigen receptor (CAR) T-cell therapies, which have shown encouraging results in preclinical and early-phase clinical trials. Despite their promise, challenges such as drug resistance and toxicity must be addressed before these therapies can become mainstream treatments for SCLC. The future of DLL3 as a therapeutic target holds great potential, offering hope for improved survival outcomes and reduced side effects for SCLC patients. Continued research and clinical trials are crucial in realizing its full potential as a transformative treatment for SCLC.
Introduction
Small cell lung cancer (SCLC) accounts for approximately 15% of all lung cancer cases, but despite its relatively smaller prevalence, it is one of the deadliest forms of cancer. The high mortality rate associated with SCLC is a direct consequence of its aggressive nature, rapid progression, and tendency to metastasize early. SCLC is often diagnosed in the later stages, making treatment options less effective and survival rates significantly low.
While first-line treatments such as chemotherapy and radiation therapy have been somewhat effective in controlling early-stage SCLC, most patients experience a relapse within a year or two. The disease often becomes refractory to conventional therapies, leading to poor prognoses. Current treatment options for advanced or recurrent SCLC are limited, with few breakthroughs in improving survival rates in these cases. Moreover, the harsh side effects and toxicities of these treatments only add to the burden, severely impacting patients’ quality of life.
Despite advancements in understanding the molecular mechanisms underlying SCLC, there has been little progress in developing targeted therapies that specifically address the unique biology of this cancer. This gap highlights a pressing need for novel treatment approaches that can effectively combat the disease without causing severe side effects.
One such promising target is Delta-like ligand 3 (DLL3), a protein that is highly expressed in SCLC and other neuroendocrine tumors but minimally present in normal tissues. DLL3 plays a crucial role in the Notch signaling pathway, which is often dysregulated in SCLC, making it an attractive candidate for targeted therapy. The selective expression of DLL3 in tumor cells has led to the development of several DLL3-targeted therapies, which may provide new hope for patients with SCLC.
In the following sections, we will explore DLL3 in greater detail and discuss the emerging therapeutic strategies being developed to target this protein in SCLC.
What is Delta-Like Ligand 3 (DLL3) and Why Does It Matter in SCLC?
Delta-like ligand 3 (DLL3) is an inhibitory ligand of the Notch signaling pathway, a critical pathway involved in cell differentiation, proliferation, and apoptosis. DLL3’s unique expression profile makes it an intriguing candidate for targeted cancer therapies, especially in small cell lung cancer (SCLC). Unlike normal tissues, which exhibit minimal DLL3 expression, SCLC cells show high levels of DLL3, making it a potential target for therapeutic intervention.
The Notch signaling pathway is essential for maintaining cellular identity and homeostasis. However, in SCLC, this pathway is often dysregulated, contributing to uncontrolled cell growth and resistance to normal cell death processes. DLL3 acts by binding to the Notch receptors and preventing their activation, thereby blocking normal Notch signaling in tumor cells. This disruption is part of the oncogenic process in neuroendocrine tumors like SCLC.
DLL3’s limited expression in normal tissues means that therapies targeting DLL3 can potentially have minimal off-target effects, reducing the toxicities often associated with cancer treatments. This selective expression pattern makes DLL3 an attractive therapeutic target for SCLC, where there is a dire need for more effective and less toxic treatment options.
In addition to its role in SCLC, DLL3 is also expressed in other neuroendocrine tumors, including certain types of small-cell neuroendocrine carcinoma of the prostate and other rare cancers. Understanding DLL3’s role in these cancers could open the door to broader therapeutic applications, offering a potential multi-cancer strategy.
As research into DLL3 continues to evolve, its role as a key driver of SCLC malignancy makes it a focal point in the development of targeted therapies for this aggressive cancer.
Therapeutic Strategies Targeting DLL3: A Game-Changer for SCLC?
The discovery of DLL3 as a promising target for small cell lung cancer (SCLC) has led to the development of several innovative therapeutic strategies. DLL3’s selective expression in SCLC cells offers a unique opportunity to target the cancer directly while minimizing damage to healthy tissues. Three major therapeutic approaches being explored to target DLL3 include antibody-drug conjugates (ADC), bispecific T-cell engagers (BiTE), and chimeric antigen receptor (CAR) T-cell therapy.
Antibody-Drug Conjugates (ADC): Rovalpituzumab tesirine is a potent ADC designed to specifically target DLL3-expressing SCLC cells. This therapy involves an antibody that binds to DLL3, delivering a cytotoxic drug directly to the cancer cells. Rovalpituzumab tesirine has shown promising results in early-phase clinical trials, with some patients experiencing durable responses, providing hope for more effective treatment of advanced SCLC.
Bispecific T-cell Engagers (BiTE): AMG757 is a bispecific T-cell engager that brings T cells into close proximity to DLL3-expressing cancer cells. By binding both to DLL3 on the tumor cell and to the T-cell receptor, AMG757 activates the patient’s immune system to attack the tumor. Early studies suggest that AMG757 has potential for treating SCLC, offering an immune-based approach to tackle the disease more effectively.
Chimeric Antigen Receptor (CAR) T-cell Therapy: Another cutting-edge strategy is CAR T-cell therapy, which involves modifying a patient’s own T cells to express a receptor targeting DLL3. These engineered T cells are then infused back into the patient, where they can specifically seek out and destroy SCLC cells. AMG119 is one such CAR T-cell therapy under development, and initial trials have shown encouraging results.
Preclinical and Clinical Evidence for DLL3-Targeted Therapies
The preclinical and clinical evidence supporting DLL3-targeted therapies for small cell lung cancer (SCLC) has grown significantly, showing promising results and paving the way for new treatment options. Researchers have explored various strategies to target DLL3, including antibody-drug conjugates (ADC), bispecific T-cell engagers (BiTE), and chimeric antigen receptor (CAR) T-cell therapies. Preclinical studies have provided crucial insights into the effectiveness of these treatments in targeting DLL3-expressing SCLC cells.
In preclinical models, DLL3-targeted therapies have demonstrated potent anti-tumor activity, highlighting the potential of these treatments in eliminating SCLC cells while sparing normal tissues. For example, rovalpituzumab tesirine, an ADC, showed significant tumor regression in SCLC xenograft models by specifically delivering a cytotoxic agent to DLL3-expressing cells. This therapy’s success in preclinical trials laid the foundation for subsequent clinical studies.
In the clinical setting, DLL3-targeted therapies have entered early-phase clinical trials. One of the most notable examples is rovalpituzumab tesirine, which has shown promise in patients with heavily pretreated SCLC. In a Phase 1 clinical trial, patients receiving rovalpituzumab tesirine experienced durable responses, and the therapy was well-tolerated with manageable side effects. Other therapies, like the bispecific T-cell engager AMG757, have also shown encouraging early-phase results, with some patients experiencing clinical benefits.
Although these therapies are still in the early stages of clinical testing, the results so far are promising. The selective expression of DLL3 in SCLC cells, combined with the effectiveness of these novel treatments in preclinical models and early-phase clinical trials, provides hope that DLL3-targeted therapies will become a cornerstone in the management of SCLC.
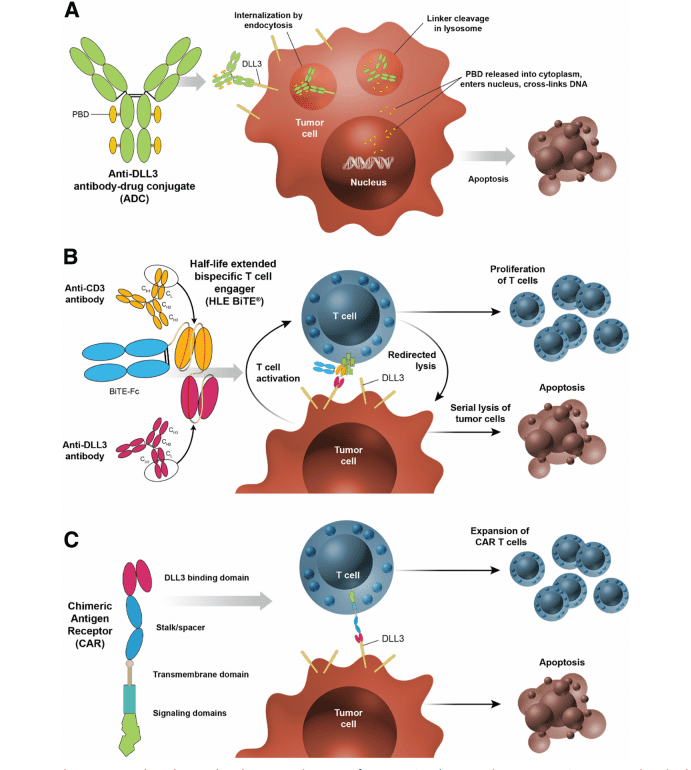
Fig. 1 DLL3-targeted investigational products utilize distinct mechanisms of action.
As clinical trials continue, researchers are optimistic that DLL3-targeted therapies will become a valuable addition to the limited arsenal of treatments available for SCLC.
The Future of DLL3 in SCLC Treatment: Challenges and Opportunities
While DLL3-targeted therapies offer significant promise for improving outcomes in small cell lung cancer (SCLC), several challenges must be addressed before they can become a mainstay in clinical practice. These challenges include issues related to drug resistance, the potential for off-target effects, and the overall safety profile of these therapies.
One major challenge is the development of resistance. Like many cancer treatments, DLL3-targeted therapies may face resistance mechanisms that limit their long-term effectiveness. In some cases, SCLC tumors may adapt by downregulating DLL3 expression or altering the Notch signaling pathway to bypass the targeted inhibition. Overcoming resistance will require continued research into combination therapies and alternative approaches to target DLL3 in more resilient tumor cells.
Another concern is the toxicity of DLL3-targeted treatments. Although these therapies show promising selectivity for cancer cells, the risk of off-target effects remains a concern, especially since some normal tissues may also express low levels of DLL3. Managing these potential side effects will be crucial for the widespread use of DLL3-targeted therapies, especially in patients who have already undergone multiple rounds of chemotherapy or radiation therapy.
Despite these challenges, the opportunities for DLL3-targeted therapies are vast. As research progresses, combining DLL3-targeted therapies with other treatment modalities, such as immune checkpoint inhibitors, could enhance their effectiveness and reduce the likelihood of resistance. Moreover, the development of more selective drugs and the identification of biomarkers for patient selection may help optimize treatment strategies, minimizing side effects and improving patient outcomes.
Looking ahead, DLL3-targeted therapies could not only revolutionize the treatment of SCLC but also pave the way for broader applications in other neuroendocrine tumors. Continued investment in clinical trials and preclinical studies will be essential to fully realize the potential of DLL3 as a therapeutic target.
References
- Owen, D. H., Giffin, M. J., Bailis, J. M., Smit, M.-A. D., Carbone, D. P., & He, K. (2019). DLL3: An emerging target in small cell lung cancer. Journal of Hematology & Oncology, 12, 61. DOI
- Carbone, D. P., & Hirsch, F. R. (2018). Small cell lung cancer: Current concepts and future directions. Lung Cancer, 123, 1-11. DOI
- Gazdar, A. F. (2016). Targeted therapy for small cell lung cancer. Frontiers in Oncology, 6, 10-15. DOI
- van Zandwijk, N., & Jassem, J. (2018). Small cell lung cancer: Where are we now? European Respiratory Review, 27(148), 1-8. DOI
- Rudin, C. M., & Poirier, J. T. (2020). The emerging role of immunotherapy in small cell lung cancer. Journal of Clinical Oncology, 38(23), 2862-2873. DOI
- Kummar, S., & Kaur, R. (2017). Delta-like ligand 3: A promising target for small cell lung cancer therapy. Oncology Reports, 37(6), 3170-3179. DOI