Targeting DNA Damage Checkpoint Kinases: A New Frontier in Precision Cancer Therapy
Abstract
DNA damage checkpoint kinases, specifically the ATM/CHK2 and ATR/CHK1/WEE1 pathways, are essential components of the DNA damage response (DDR) and play a critical role in maintaining genomic integrity by arresting the cell cycle for DNA repair or triggering apoptosis in cases of severe damage. In cancer cells, mutations in these pathways can lead to uncontrolled cell proliferation and tumor development. Targeted inhibition of these kinases has emerged as a promising therapeutic strategy, particularly in tumors with DNA repair deficiencies, as it can induce lethal DNA damage selectively in cancer cells. Current research focuses on developing and optimizing checkpoint kinase inhibitors, such as those targeting ATR, CHK1, and WEE1, which are in clinical trials for various cancers. These inhibitors are being tested alone and in combination with DNA-damaging agents and immune checkpoint inhibitors to enhance anti-tumor effects. However, challenges remain, including identifying predictive biomarkers and overcoming resistance mechanisms. The potential of DDR inhibitors in combination therapies underscores their promising role in precision cancer treatment.
Introduction to DNA Damage Checkpoint Kinases
DNA damage response (DDR) is a critical mechanism in cellular defense, responsible for detecting and repairing DNA damage to maintain genomic stability. Cells encounter various forms of DNA damage daily from environmental factors like UV light and chemicals, as well as from normal metabolic processes. When this damage goes uncorrected, it can lead to mutations, genome instability, and potentially cancerous transformations. DDR pathways, therefore, act as a cellular surveillance system to preserve DNA integrity and prevent oncogenesis.
Central to the DDR are the DNA damage checkpoint kinases, a group of enzymes that monitor and repair damaged DNA by coordinating cell cycle checkpoints with repair mechanisms. Two major DDR pathways are mediated by ATM/CHK2 and ATR/CHK1/WEE1 kinases. These kinases sense damage and relay signals to halt the cell cycle, allowing time for DNA repair or triggering apoptosis if the damage is irreparable. The ATM/CHK2 pathway predominantly manages the G1/S checkpoint, halting the cycle before DNA synthesis begins, while ATR/CHK1/WEE1 controls the S and G2/M checkpoints, responding to damage occurring during DNA replication or before cell division.
In the context of cancer, these pathways play a dual role. On one hand, they protect cells from accumulating mutations by repairing damage and preventing genomic instability. On the other, mutations in these kinases or in their downstream effectors, such as the tumor suppressor protein p53, can disable cell cycle control, allowing cancer cells to proliferate unchecked. Loss of function in DDR components, especially in cancer types with high mutation rates, opens new avenues for targeted therapies. Drugs that inhibit ATM or ATR pathways, for instance, can exploit the cancer cells’ compromised repair systems, making them more vulnerable to DNA-damaging agents.
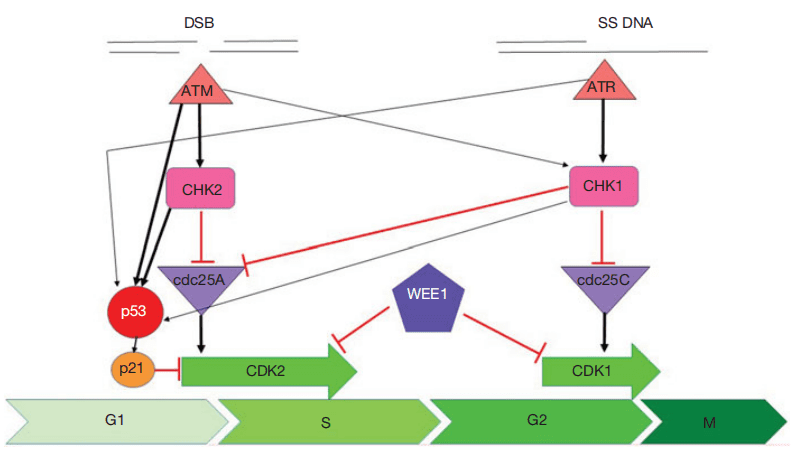
Fig. 1. Cell cycle checkpoint signalling.
Thus, DDR checkpoint kinases are essential to both cancer prevention and treatment strategies, with ongoing research focused on leveraging these pathways to improve therapeutic outcomes.
ATM/CHK2 and ATR/CHK1/WEE1 Pathways in Cancer
The ATM/CHK2 and ATR/CHK1/WEE1 pathways are central to the cell cycle’s response to DNA damage, with each pathway responsible for activating specific checkpoints that prevent the propagation of damaged DNA. These pathways detect distinct types of DNA lesions and initiate targeted responses. The ATM/CHK2 pathway is primarily activated by double-strand DNA breaks (DSBs), which can arise from factors like ionizing radiation or chemotherapeutic agents. Upon detecting a DSB, the ATM kinase is recruited to the damage site and activates CHK2. This activation initiates a cascade of phosphorylation events, leading to the stabilization of p53, a key tumor suppressor protein. Stabilized p53 triggers the transcription of genes that either arrest the cell cycle at the G1/S checkpoint or initiate apoptosis if the damage is extensive and irreparable. Thus, the ATM/CHK2 pathway plays a critical role in preventing the replication of damaged DNA.
The ATR/CHK1/WEE1 pathway, in contrast, is typically activated by single-stranded DNA (ssDNA) regions that result from stalled replication forks. When ssDNA is detected, ATR is recruited and activates CHK1, leading to the phosphorylation of WEE1. This series of events prevents the entry into mitosis by arresting the cell cycle at the S and G2/M checkpoints, allowing time for repair processes to resolve the replication stress. In cancer, defects in either of these pathways—especially through mutations in p53, ATM, or ATR—can disable these checkpoints and allow cells to proliferate despite accumulating damage, contributing to tumor growth and survival.
The ATM/CHK2 and ATR/CHK1/WEE1 pathways are not entirely independent; they exhibit significant cross-talk. For instance, CHK1, although primarily activated by ATR, can also be phosphorylated by ATM in response to DSBs. This redundancy ensures robust protection against a range of DNA insults. However, it also highlights potential vulnerabilities in cancer cells, where targeted inhibition of these pathways can make tumor cells more susceptible to DNA-damaging treatments, as their repair mechanisms and checkpoints are already compromised.
Pathway Dysfunctions and Cancer Development
The ATM/CHK2 and ATR/CHK1/WEE1 pathways are critical to maintaining genome integrity. Mutations or dysfunctions in these pathways can lead to unchecked cell division and cancer development. In healthy cells, these pathways respond to DNA damage by arresting the cell cycle to enable repair or, if damage is severe, initiating apoptosis. However, when mutations occur in key components, such as ATM, CHK2, ATR, CHK1, or the tumor suppressor p53, the cell’s ability to manage DNA damage is compromised, allowing mutations to accumulate and leading to genome instability—a hallmark of cancer.
The ATM gene, which senses double-strand DNA breaks (DSBs), is frequently mutated in cancers, particularly in breast, lung, and hematologic malignancies. Mutations in ATM result in defective G1/S checkpoint control, allowing cells with DSBs to enter the S phase and propagate errors. Loss of ATM function is also linked to ataxia-telangiectasia, a genetic disorder characterized by increased cancer risk due to a compromised DNA damage response. Similarly, mutations in CHK2, the downstream effector of ATM, impair cell cycle arrest and apoptosis. CHK2 mutations are linked to Li-Fraumeni syndrome, a hereditary cancer predisposition disorder, as well as to sporadic cancers such as those of the breast, colon, and prostate.
The ATR/CHK1/WEE1 pathway, which is activated by single-stranded DNA and replication stress, also plays a pivotal role in tumor suppression. Dysfunction in this pathway is less common but highly consequential. Loss of ATR function, often due to hypomorphic mutations, can lead to Seckel syndrome and has been associated with increased tumor incidence. Additionally, elevated levels of CHK1 and WEE1 are often observed in aggressive cancers, where they support rapid cell proliferation by managing replication stress, yet their inhibition can sensitize cancer cells to DNA-damaging therapies.
Together, these pathway dysfunctions contribute to cancer by promoting DNA replication without proper repair mechanisms, allowing mutations to persist and cells to divide uncontrollably. Exploiting these vulnerabilities is a promising therapeutic strategy, especially for cancers with inherent checkpoint deficiencies.
Therapeutic Targeting of Checkpoint Kinases
The ATM/CHK2 and ATR/CHK1/WEE1 pathways have become valuable targets in cancer therapy due to their roles in DNA damage response and cell cycle control. In cancers where these pathways are partially compromised, further inhibition can exploit the cancer cells’ reduced capacity for DNA repair, leading to selective cell death. This strategy, known as synthetic lethality, involves targeting checkpoint kinases in tumor cells that have existing deficiencies in DNA repair genes, such as mutations in BRCA1/2 or p53, making them particularly susceptible to DNA damage.
ATM inhibitors have shown promise in enhancing the effects of radiation and certain chemotherapeutics that induce double-strand breaks (DSBs). ATM inhibition sensitizes cancer cells to DNA-damaging agents, which are more effective when DNA repair is impaired. Although early ATM inhibitors faced issues with poor bioavailability, newer compounds like AZD0156 have demonstrated improved properties and are now in clinical trials for their effectiveness in combination with other therapies.
CHK1 and CHK2 inhibitors also hold potential as cancer treatments. CHK1 inhibition is particularly effective in cells with high replication stress, a common feature of many tumors. CHK1 inhibitors like MK-8776 and prexasertib have shown to enhance the cytotoxic effects of DNA-damaging agents, especially in tumors with compromised p53 function. CHK2 inhibitors, while fewer in development, could prove useful in specific cancers with ATM and p53 deficiencies, where CHK2 loss leads to an even greater vulnerability to DNA damage.
WEE1 inhibitors, such as AZD1775, prevent cells from pausing the cell cycle for repair, forcing cells with damaged DNA to enter mitosis prematurely, leading to cell death. This approach is particularly effective in p53-mutant cancers that lack a functional G1 checkpoint. WEE1 inhibition has shown efficacy in combination with traditional chemotherapeutics like cisplatin and gemcitabine, and WEE1 inhibitors are being investigated in several clinical trials.
These targeted checkpoint kinase inhibitors offer a promising approach to cancer treatment, particularly when combined with DNA-damaging agents, enhancing efficacy while potentially reducing the required doses of traditional therapies.
Future Directions and Clinical Implications
Targeting DNA damage response (DDR) pathways, specifically the ATM/CHK2 and ATR/CHK1/WEE1 pathways, presents a promising direction for precision cancer therapy. While these checkpoint kinases play a critical role in maintaining genomic stability, their dysfunction in cancer cells can be exploited therapeutically. By inhibiting these pathways, especially in tumors with existing DNA repair deficiencies, cancer therapies can effectively induce lethal DNA damage selectively in tumor cells, sparing normal cells. This approach is particularly relevant for cancers with mutations in DDR-related genes like BRCA1/2, TP53, or ATM, which make cancer cells reliant on alternative repair mechanisms that can be therapeutically targeted.
Checkpoint kinase inhibitors are currently being tested in combination with other therapies. For instance, ATR and WEE1 inhibitors are effective in tumors with high levels of replication stress, such as those driven by oncogenes like MYC. These inhibitors can potentiate the effects of chemotherapy or radiation, which rely on inducing DNA damage to eliminate cancer cells. Clinical trials are underway to assess the efficacy of ATR inhibitors like AZD6738 and CHK1 inhibitors such as prexasertib in various cancers, including ovarian, pancreatic, and lung cancers.
Looking ahead, one challenge in the clinical application of DDR inhibitors is identifying biomarkers that can predict treatment response. Biomarkers for pathway mutations or repair deficiencies, like BRCA mutation status or p53 functionality, can help personalize therapy, ensuring that only patients likely to benefit are treated with DDR inhibitors. Additionally, understanding resistance mechanisms is crucial, as some cancers may develop compensatory responses that bypass DDR inhibition, leading to therapeutic resistance.
Another promising area is combining DDR inhibitors with immune checkpoint inhibitors. DNA damage can generate neoantigens and activate immune responses, providing a rationale for dual targeting of DDR and immune checkpoints. Trials are examining combinations like ATR and PARP inhibitors with PD-1/PD-L1 blockers, exploring a synergistic approach to enhance anti-tumor immunity. These combined approaches hold promise for creating more durable responses in cancer therapy.
References
- Smith, H. L., Southgate, H., Tweddle, D. A., & Curtin, N. J. (2020). DNA damage checkpoint kinases in cancer. Expert Reviews in Molecular Medicine, 22, e2.
- Shiloh, Y., & Ziv, Y. (2013). The ATM protein kinase: Regulating the cellular response to genotoxic stress, and more. Nature Reviews Molecular Cell Biology, 14(4), 197–210.
- Blackford, A. N., & Jackson, S. P. (2017). ATM, ATR, and DNA-PK: The trinity at the heart of the DNA damage response. Molecular Cell, 66(6), 801–817.
- Brown, J. S., O’Carrigan, B., Jackson, S. P., & Yap, T. A. (2017). Targeting DNA repair in cancer: Beyond PARP inhibitors. Cancer Discovery, 7(1), 20–37.
- Vendetti, F. P., et al. (2015). The orally active and bioavailable ATR kinase inhibitor AZD6738 potentiates the anti-tumor effects of cisplatin to resolve ATM-deficient non-small cell lung cancer in vivo. Oncotarget, 6(6), 44289–44305.
- Hickson, I., et al. (2004). Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Research, 64(24), 9152–9159.
- Weber, A. M., & Ryan, A. J. (2015). ATM and ATR as therapeutic targets in cancer. Pharmacology & Therapeutics, 149, 124–138.
- Kitao, H., & Yoshida, K. (2018). DNA replication stress and cancer chemotherapy. Cancer Science, 109(2), 264–271.




