Targeting Myeloid-Derived Suppressor Cells (MDSCs) through CXCR1/2 Inhibition: The Potential of SX-682 in Cancer Immunotherapy
Abstract
SX-682 is a small molecule allosteric inhibitor of CXCR1/2, which has shown potential as a therapeutic agent for these diseases by inhibiting leukocyte migration and inflammation via blocking the binding of chemokines to CXCR1/2. In particular, SX-682 has been studied for its ability to inhibit the trafficking of myeloid-derived suppressor cells (MDSCs), a subset of immune cells that can suppress T cell-mediated antitumor immunity. This has led to investigations of SX-682 in combination with other immunotherapies, such as natural killer (NK) cell or T cell immunotherapy, with promising preclinical results.
Myeloid-derived suppressor cells (MDSCs) play a role in tumor progression and suppress NK cell-mediated antitumor immunity
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells that accumulate in the tumor microenvironment and peripheral blood of cancer patients. MDSCs are known to play a critical role in tumor progression by suppressing immune responses against cancer cells, including NK cell-mediated antitumor immunity.
MDSCs exert their suppressive effects through several mechanisms, including the production of reactive oxygen species, nitric oxide, and arginase. These molecules impair the function of NK cells by reducing their cytotoxicity and cytokine production. MDSCs also inhibit NK cell activation by modulating the expression of activating and inhibitory receptors on their surface.
In addition to their direct effects on NK cells, MDSCs also promote the expansion of regulatory T cells (Tregs), which further suppress immune responses against cancer cells. Tregs can inhibit NK cell function by secreting immunosuppressive cytokines and modulating the expression of activating and inhibitory receptors on their surface.
The accumulation of MDSCs in the tumor microenvironment is thought to be driven by factors released by cancer cells, including cytokines, chemokines, and growth factors. These factors activate the signaling pathways that promote the expansion and differentiation of MDSCs. Additionally, MDSCs can be recruited to the tumor microenvironment through chemotaxis mediated by chemokines such as CXCL8 and CXCL12.
The suppressive effects of MDSCs on NK cell-mediated antitumor immunity make them an attractive target for cancer immunotherapy. Several approaches have been developed to target MDSCs, including inhibitors of their suppressive pathways and depletion strategies. In particular, inhibitors of CXCR1/2 signaling, such as SX-682, have shown promising results in preclinical studies by inhibiting the trafficking of MDSCs to the tumor microenvironment.
In summary, MDSCs play a critical role in tumor progression by suppressing NK cell-mediated antitumor immunity through various mechanisms. Targeting MDSCs may be a promising approach to enhance NK cell-mediated antitumor immunity and improve cancer immunotherapy outcomes.
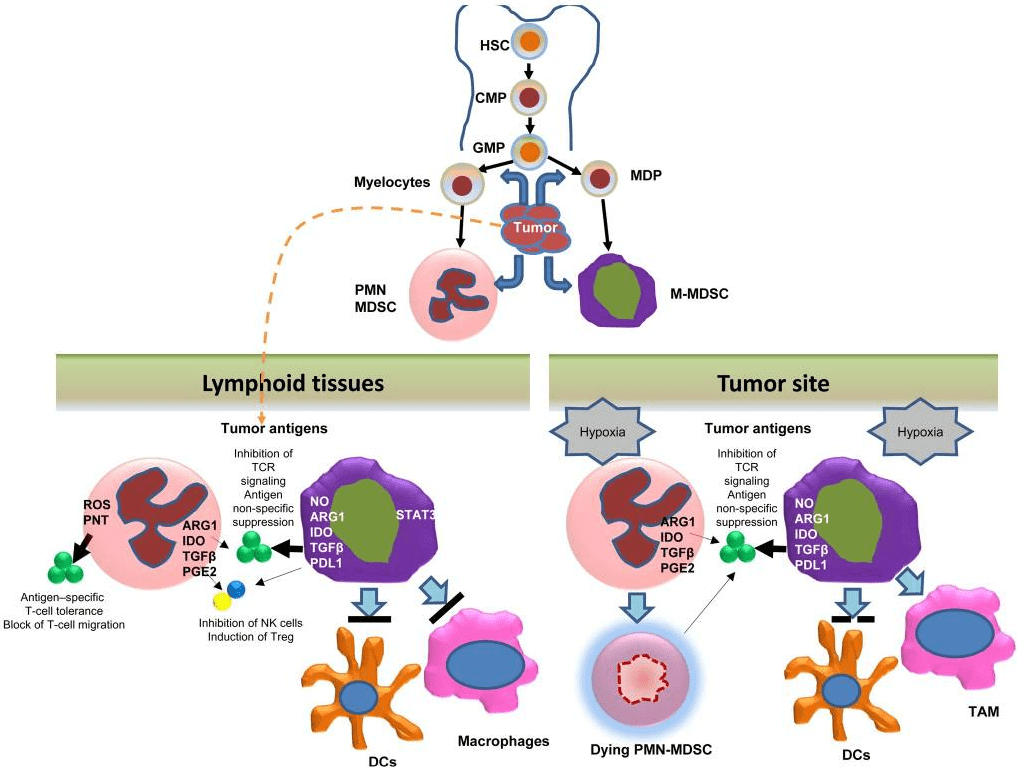
Fig 1. Development and function of MDSC[1].
CXCR1 and CXCR2 are involved in MDSC trafficking
CXCR1 and CXCR2 are chemokine receptors that are involved in the recruitment and trafficking of myeloid-derived suppressor cells (MDSCs) to the tumor microenvironment. CXCR1 binds to CXCL6 and CXCL8, while CXCR2 binds to CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8. These chemokines are often overexpressed in the tumor microenvironment and attract MDSCs to the site of the tumor.
The activation of CXCR1 and CXCR2 signaling pathways leads to the upregulation of adhesion molecules on MDSCs, such as integrins and selectins, which facilitate their attachment to the endothelium and subsequent transmigration into the tumor microenvironment. Once inside the tumor, MDSCs can suppress immune responses against cancer cells through various mechanisms.
Inhibitors of CXCR1/2 signaling, such as SX-682, have shown promising results in preclinical studies by inhibiting the trafficking of MDSCs to the tumor microenvironment. This highlights the potential of targeting CXCR1 and CXCR2 as a strategy to enhance the efficacy of cancer immunotherapy by preventing the recruitment of MDSCs to the tumor microenvironment.
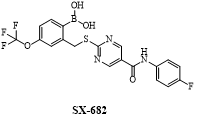
Fig 2. The structure of SX-682
The action mechanism of SX-682
SX-682 is a small molecule inhibitor of CXCR1 and CXCR2, which are chemokine receptors that are involved in the recruitment and trafficking of myeloid-derived suppressor cells (MDSCs) to the tumor microenvironment. The mechanism of action of SX-682 is based on its ability to block the binding of chemokines such as CXCL8, CXCL1, and CXCL2 to CXCR1 and CXCR2.
By inhibiting CXCR1 and CXCR2 signaling pathways, SX-682 prevents the activation of downstream signaling pathways that are involved in the upregulation of adhesion molecules on MDSCs. This reduces the ability of MDSCs to attach to the endothelium and transmigrate into the tumor microenvironment, leading to a decrease in their accumulation in the tumor.
Preclinical studies have shown that SX-682 can enhance the efficacy of cancer immunotherapy by inhibiting MDSC trafficking to the tumor microenvironment and promoting NK cell-mediated antitumor immunity. This highlights the potential of SX-682 as a promising therapeutic strategy for the treatment of cancer.
SX-682 inhibits the migration of MDSCs in vitro
In vitro studies have shown that SX-682, a small molecule inhibitor of CXCR1/2, can effectively inhibit the migration of myeloid-derived suppressor cells (MDSCs). MDSCs are a heterogeneous population of immune cells that are recruited to the tumor microenvironment and suppress antitumor immunity. They play a critical role in tumor progression and resistance to immunotherapy.
In one study, treatment with SX-682 was found to significantly inhibit the chemotaxis of MDSCs towards CXCL8 in vitro, which is a chemokine involved in MDSC recruitment. SX-682 treatment also reduced the expression of integrin αLβ2, which is an adhesion molecule that facilitates MDSC attachment to endothelial cells.
These findings demonstrate the potential of SX-682 as a promising therapeutic strategy for inhibiting MDSC migration and improving the efficacy of cancer immunotherapy. Further studies are needed to investigate the efficacy and safety of SX-682 in vivo and its potential as a clinical candidate for the treatment of cancer.
SX-682 enhances NK Cell-Mediated antitumor activity in head and neck cancer models
In vivo experiments have shown that SX-682, a CXCR1/2 inhibitor, can enhance NK cell-mediated antitumor activity in head and neck cancer models. In one study, treatment with SX-682 was found to significantly decrease the number of MDSCs in the tumor microenvironment and increase the number of NK cells, leading to an improvement in the antitumor response.
Additionally, treatment with SX-682 in combination with NK cell therapy was found to be more effective than NK cell therapy alone in reducing tumor growth and improving overall survival in mouse models of head and neck cancer.
These findings suggest that SX-682 has the potential to enhance the efficacy of NK cell-based immunotherapy in the treatment of cancer. Further clinical studies are needed to evaluate the safety and efficacy of SX-682 in humans, and to determine its optimal use in combination with other immunotherapeutic agents.
Summary
The findings of this study suggest that CXCR1/2 inhibition with SX-682 has the potential to improve NK cell-mediated antitumor immunity in head and neck cancer. By targeting MDSC trafficking and reducing their presence in the tumor microenvironment, SX-682 can enhance the recruitment and activation of NK cells, leading to a more effective antitumor response.
Furthermore, combination therapy with SX-682 and NK cell immunotherapy may be a promising strategy for the treatment of head and neck cancer. The in vivo experiments demonstrated that this combination therapy was more effective than NK cell therapy alone in reducing tumor growth and improving overall survival. However, further studies are needed to optimize the dosing and scheduling of SX-682 in combination with immunotherapy to maximize its efficacy while minimizing potential side effects.
In summary, the results provide important insights into the potential of SX-682 as a therapeutic agent in the treatment of head and neck cancer. By targeting the CXCR1/2 pathway, SX-682 has the potential to enhance NK cell-mediated antitumor immunity, and combination therapy with NK cell immunotherapy may be a promising strategy for the treatment of this disease. Further studies are needed to fully evaluate the safety and efficacy of this approach and to optimize its use in clinical practice.
Reference
- Gabrilovich, D. I. (2017). Myeloid-derived suppressor cells. Cancer immunology research, 5(1), 3-8.
- Greene, S., Robbins, Y., Mydlarz, W. K., Huynh, A. P., Schmitt, N. C., Friedman, J., … & Allen, C. (2020). Inhibition of MDSC Trafficking with SX-682, a CXCR1/2 Inhibitor, Enhances NK-Cell Immunotherapy in Head and Neck Cancer ModelsMyeloid Cell Inhibition Enhances NK Cellular Immunotherapy. Clinical Cancer Research, 26(6), 1420-1431.
- Sun, L., Clavijo, P. E., Robbins, Y., Patel, P., Friedman, J., Greene, S., … & Allen, C. T. (2019). Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI insight, 4(7).
- Kargl, J., Zhu, X., Zhang, H., Yang, G. H., Friesen, T. J., Shipley, M., … & Houghton, A. M. (2019). Neutrophil content predicts lymphocyte depletion and anti-PD1 treatment failure in NSCLC. JCI insight, 4(24).
- Yang, J., Yan, C., Vilgelm, A. E., Chen, S. C., Ayers, G. D., Johnson, C. A., & Richmond, A. (2021). Targeted Deletion of CXCR2 in Myeloid Cells Alters the Tumor Immune Environment to Improve Antitumor ImmunityMyeloid-Targeted CXCR2 Deletion Improves Antitumor Immunity. Cancer immunology research, 9(2), 200-213.