Targeting Phagocytosis Checkpoints: A New Frontier in Cancer Immunotherapy
Abstract
In cancer immunotherapy, phagocytosis checkpoint blockage has shown promise as a way to target the processes that cancer cells use to avoid immune destruction. An important phagocytosis roadblock, the CD47–SIRPα axis prevents macrophages from swallowing cancer cells, allowing tumors to grow unchecked. Preclinical and early clinical trials have demonstrated great potential for therapeutic approaches that disrupt this axis. Phagocytosis checkpoint inhibitors improve both adaptive immunity and cancer cell clearance by stimulating innate immune responses and reactivating macrophages. This combination results in more potent and long-lasting anti-tumor responses. Particularly for individuals with tumors that are resistant to current treatments, the combination of these medicines with well-established ones like PD-1/PD-L1 inhibitors holds enormous potential for the advancement of cancer treatment.
Introduction
By using the body’s immune system to identify and eliminate cancerous cells, cancer immunotherapy has completely changed the way that many types of cancer are treated. The significance of innate immune checkpoints in the development of cancer has recently come to light, despite the fact that adaptive immune checkpoints like PD-1 and CTLA-4 have received a lot of attention. A crucial method by which cancer cells avoid immune identification is via the CD47–SIRPα axis, in which CD47 functions as a “don’t eat me” signal to hinder macrophage phagocytosis. In cancer therapy, targeting this axis, often referred to as a phagocytosis checkpoint, has become a viable approach for boosting immune responses. Immune cells can identify and eradicate tumor cells more effectively by inhibiting this connection, providing fresh hope in the fight against cancer.
Understanding Phagocytosis in Cancer Immunotherapy
An essential function of the immune system is phagocytosis, in which immune cells—macrophages in particular—engulf and eliminate infections or dead cells. The ability of immune cells to eradicate tumor cells is another important function of this process in cancer immunotherapy. However, by taking advantage of phagocytosis checkpoints, cancer cells have evolved highly complex strategies to avoid this immune clearance. These innate immune system-specific checkpoints work similarly to adaptive immunological checkpoints such as PD-1 and CTLA-4.
The CD47–SIRPα axis is one of the most researched phagocytosis checkpoints. Many cancer cells have an overexpression of the transmembrane protein CD47 on their surface, which binds to the SIRPα receptor on macrophages to send a “don’t eat me” signal. This interaction prevents macrophages from engaging in phagocytic activity, which permits cancer cells to elude immune surveillance. The CD47–SIRPα axis is essential for the growth of tumors because it inhibits macrophages from consuming and eliminating cancer cells.
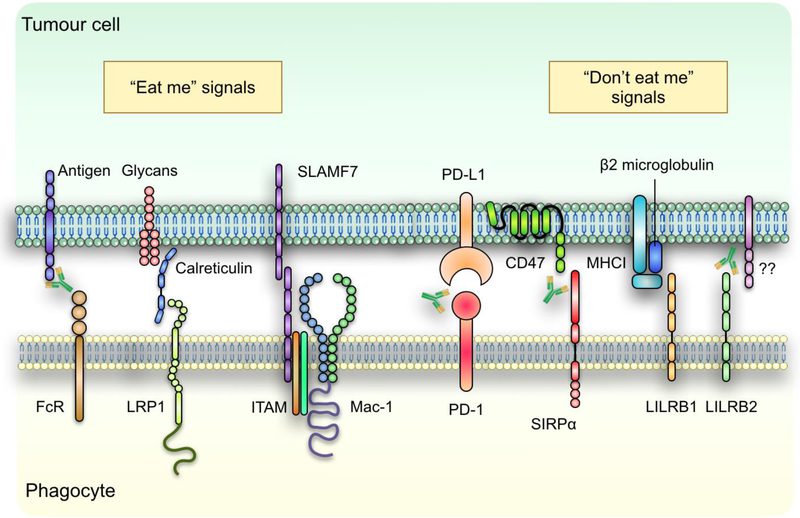
Fig. 1 | regulation of tumour cell phagocytosis.
Phagocytosis checkpoints, such the CD47–SIRPα axis, have been the focus of a novel cancer immunotherapy strategy. Macrophages can be stimulated again to identify and eliminate cancer cells by obstructing the connection between CD47 and SIRPα. Promising findings from preclinical research and early clinical trials indicate that focusing on these innate immune checkpoints may improve the efficacy of cancer immunotherapy. Moreover, phagocytosis checkpoint inhibitors may have a synergistic effect when combined with other treatments, such as PD-1/PD-L1 inhibitors, improving patient outcomes for a variety of cancer types.
The CD47–SIRPα Axis as a Target for Immunotherapy
The crucial function of the CD47–SIRPα axis in permitting cancer cells to avoid the immune system has made it a major target in the study of cancer immunotherapy. The protein CD47, which is frequently overexpressed on the surface of cancer cells, interacts with macrophages’ SIRPα to deliver a “don’t eat me” signal. This interaction stops macrophages from engaging in phagocytic activity, which stops them from engulfing and eliminating cancer cells. Because of this, immune cells can still exist and spread, even in the presence of cancer cells.
A lot of effort has been paid to targeting the CD47–SIRPα axis as a possible therapeutic approach. Reactivating the immune system to identify and eliminate tumor cells can be achieved by obstructing this connection. Numerous treatment strategies are being investigated, such as recombinant fusion proteins, small compounds that interfere with the connection, and monoclonal antibodies that block SIRPα or CD47. Preclinical research has shown that inhibiting the CD47–SIRPα axis can enhance macrophage phagocytosis of cancer cells, resulting in tumor regression. Additionally, it seems that these treatments increase the efficacy of other immunotherapies, like checkpoint inhibitors that target PD-1 or PD-L1.
Promising outcomes have been observed in the first therapeutic trials that target the CD47–SIRPα axis, with patients experiencing little or no harm. One issue is that CD47 is expressed in healthy cells as well, which raises questions about possible negative effects. Tumors can be specifically targeted since most healthy cells do not have the “eat me” signals that cancerous ones have. Therapy targeting the CD47–SIRPα axis may open up new possibilities for patients whose malignancies are not responding to conventional treatments as research in this area progresses.
Emerging Therapies Targeting Phagocytosis Checkpoints
Treatments aimed at phagocytosis checkpoints, namely the CD47–SIRPα axis, have demonstrated noteworthy promise in the management of cancer. Through the manipulation of the CD47–SIRPα connection, these treatments facilitate the ability of macrophages and other phagocytic cells to identify and eradicate cancerous cells. Clinical trials are now being conducted on monoclonal antibodies, including Hu5F9-G4, which are designed to target CD47, for a variety of malignancies, including solid tumors and lymphoma. Furthermore, recombinant proteins have demonstrated encouraging preclinical outcomes. One such protein is SIRPαFc, which competes with CD47 for SIRPα binding.
One important area of investigation is the combination of CD47–SIRPα axis inhibition with other cancer treatments. For example, treatments targeting the CD47–SIRPα axis have shown increased anti-tumor effects when coupled with PD-1 or PD-L1 inhibitors. The combined effect of stimulating both the innate and adaptive immune systems results in a more thorough immunological assault on cancerous cells. These combinations are now being investigated in clinical studies; preliminary results indicate that individuals with advanced malignancies may benefit from them.
Other putative phagocytosis checkpoints, like MHC-I and LILRB1, are also being studied as potential therapeutic targets in addition to CD47. By preventing these connections, immune cells may be better able to identify and destroy cancer cells. To increase the impact of checkpoint inhibitors, methods for increasing the expression of pro-phagocytic signals on tumor cells, such as calreticulin, are also being investigated. Particularly for individuals who do not react to conventional therapy, these therapies give promise for more effective cancer treatments as they go through clinical trials.
The Future of Cancer Treatment: Bridging Innate and Adaptive Immunity
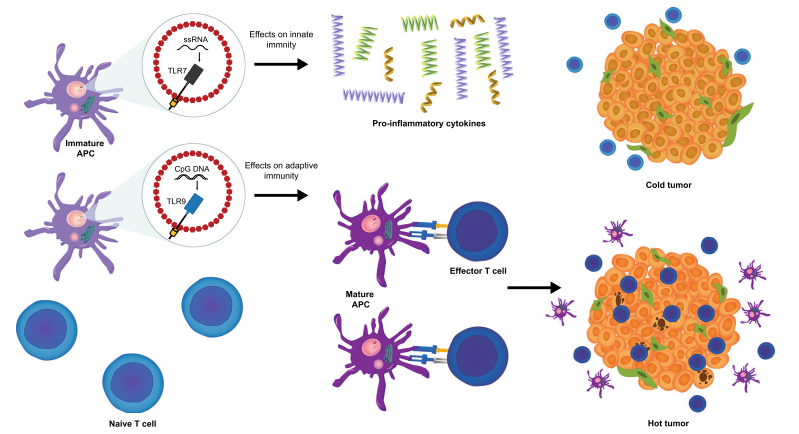
A novel approach to cancer immunotherapy is phagocytosis checkpoint blockage, which has the ability to stimulate the innate and adaptive immune systems for a more thorough anti-tumor response. By engulfing and delivering antigens to T cells, the innate immune system, principally through the action of macrophages and dendritic cells, is responsible for beginning immunological responses. Checkpoints for phagocytosis, like the CD47–SIRPα axis, obstruct this process, enabling cancer cells to evade immune recognition. In addition to reactivating macrophages to consume tumor cells, bypassing these checkpoints improves dendritic cells’ capacity to present tumor antigens, so preparing T cells for a more potent adaptive immune response.
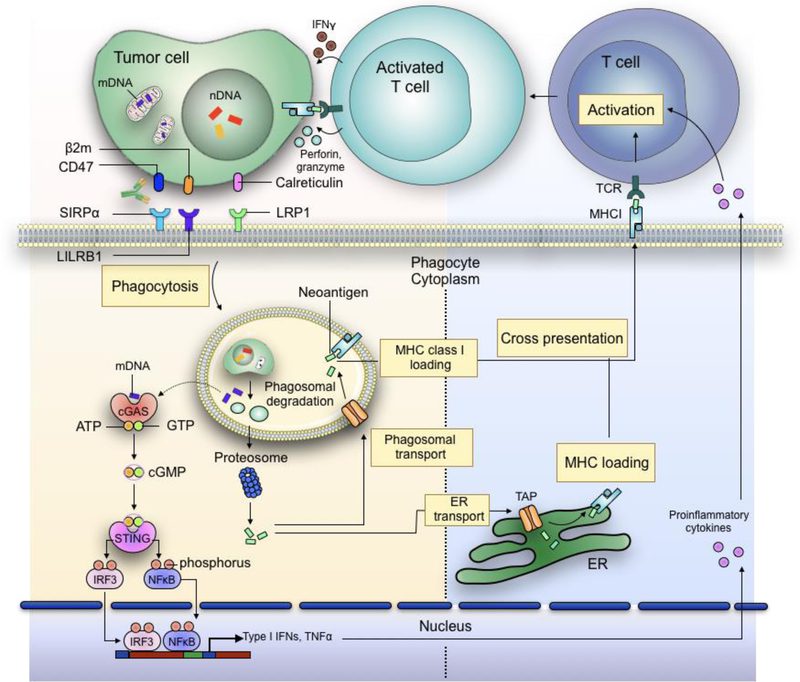
Fig. 2 | Bridging innate and adaptive antitumour immunity.
The potential for enhancing the effectiveness of cancer immunotherapies stems from this merging of innate and adaptive immunity. According to preclinical research, CD47 inhibition can improve tumor antigen cross-presentation, which will activate cytotoxic CD8+ T cells. Then, by identifying and eradicating cancer cells, these T cells provide long-term protection and lower the risk of tumor recurrence. Furthermore, phagocytosis checkpoint inhibitors might enhance anti-tumor immune responses when combined with other adaptive immune checkpoint medications, such as PD-1 or CTLA-4 inhibitors, which may result in better long-lasting therapeutic effects.
The development of next-generation cancer therapeutics is anticipated to heavily depend on the integration of innate and adaptive immune system modulation as research into the function of phagocytosis checkpoints progresses. This approach has the potential to revolutionize the field of treatment, especially for malignancies that are incurable.
Conclusion
Blocking phagocytosis checkpoints, specifically focusing on the CD47–SIRPα axis, is a promising development in cancer immunotherapy. Macrophages have the ability to identify and eradicate cancer cells more efficiently by interfering with this immune evasion process. This offers patients, particularly those who do not respond well to conventional medicines, an alternative therapeutic option. Stronger and longer-lasting anti-tumor effects result from this method, which not only strengthens innate immune responses but also closes the gap between innate and adaptive immunity.
Phagocytosis checkpoint inhibitors have shown promise in early clinical trials when used alone or in conjunction with other immunotherapies such as PD-1/PD-L1 inhibitors. These treatments have demonstrated potential for treating a variety of cancers, including solid tumors and hematological malignancies. There are still issues to be resolved, such as controlling any possible negative effects and guaranteeing selectivity for cancer cells.
Phagocytosis checkpoint inhibitors are expected to be a key component of cancer treatment in the future as research progresses. Their capacity to activate both immune systems may provide patients with treatment-resistant malignancies with new hope for long-lasting responses and remission, thus improving their prognosis.
References
- Feng, M., Jiang, W., Kim, B. Y. S., Zhang, C. C., Fu, Y. X., & Weissman, I. L. (2019). Phagocytosis checkpoints as new targets for cancer immunotherapy. Nature Reviews Cancer, 19(10), 568-586.
- Chen, Q., Zhang, X. H., & Massagué, J. (2011). Macrophage binding to receptor-regulatory proteins: Immune escape mechanism in cancer. Cell, 144(5), 707-719.
- Weiskopf, K., & Weissman, I. L. (2015). Macrophages are critical effectors of antibody therapies for cancer. Proceedings of the National Academy of Sciences, 112(29), 9261-9266.
- Oldenborg, P. A., Zheleznyak, A., Fang, Y. F., Lagenaur, C. F., Gresham, H. D., & Lindberg, F. P. (2000). Role of CD47 as a marker of self on red blood cells. Science, 288(5473), 2051-2054.
- Chao, M. P., Weissman, I. L., & Majeti, R. (2012). The CD47-SIRPα pathway in cancer immune evasion and potential therapeutic implications. Current Opinion in Immunology, 24(2), 225-232.
- Liu, X., Pu, Y., & Cron, K. (2018). CD47 blockade triggers phagocytosis and eliminates tumor cells. Cancer Research, 78(4), 1264-1272.
- Jaiswal, S., Jamieson, C. H., Pang, W. W., Park, C. Y., Chao, M. P., Majeti, R., & Weissman, I. L. (2009). CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell, 138(2), 271-285.
- Tseng, D., Volkmer, J. P., Willingham, S. B., Contreras-Trujillo, H., Fathman, J. W., Fernhoff, N. B., … & Weissman, I. L. (2013). Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proceedings of the National Academy of Sciences, 110(27), 11103-11108.