Targeting the Pentose Phosphate Pathway: A Metabolic Vulnerability in Cancer Therapy
Abstract
The Pentose Phosphate Pathway (PPP) plays a critical role in cancer cell metabolism, supporting their rapid growth and survival by providing essential metabolic intermediates and a robust antioxidant defense. This pathway consists of two phases: the oxidative phase, which produces NADPH for redox balance and biosynthetic needs, and the nonoxidative phase, which generates ribose-5-phosphate for nucleotide synthesis. In cancer cells, PPP regulation is mediated by tumor suppressors and oncogenes, with many cancers showing increased PPP activity that promotes resilience against oxidative stress and treatment resistance. Targeting the PPP has emerged as a promising therapeutic strategy, with several inhibitors currently under investigation for their ability to disrupt cancer cell metabolism. Future directions focus on developing selective PPP inhibitors with minimal toxicity and combining these with conventional treatments to enhance efficacy. This strategy could pave the way for new cancer therapies that exploit the metabolic vulnerabilities of tumors, especially in cases of treatment-resistant cancers.
Introduction to Cancer Metabolism and the Pentose Phosphate Pathway
Cancer cells exhibit a distinct metabolic behavior, enabling them to sustain rapid proliferation and survive in diverse environments. Unlike normal cells, cancer cells preferentially rely on aerobic glycolysis, a phenomenon known as the Warburg effect. This adaptation allows them to generate energy through the breakdown of glucose into lactate, even in the presence of oxygen. This metabolic shift is not solely about energy production; it enables cancer cells to accumulate essential metabolic intermediates required for their growth. By adopting aerobic glycolysis, cancer cells prioritize the synthesis of biomolecules needed for rapid cell division over maximal ATP generation.
The Pentose Phosphate Pathway (PPP) is one of the critical metabolic pathways enhanced in cancer cells to support these demands. The PPP branches off from glycolysis and operates in two phases: oxidative and nonoxidative. In the oxidative phase, the PPP generates nicotinamide adenine dinucleotide phosphate (NADPH), which plays a crucial role in maintaining redox balance by neutralizing reactive oxygen species (ROS). Additionally, NADPH serves as a reducing agent in biosynthetic pathways, including fatty acid and nucleotide synthesis, both essential for rapidly proliferating cancer cells. The nonoxidative phase of the PPP produces ribose-5-phosphate, a precursor for nucleotide synthesis that fuels DNA replication and RNA synthesis, further supporting the increased cellular demand in tumor cells.
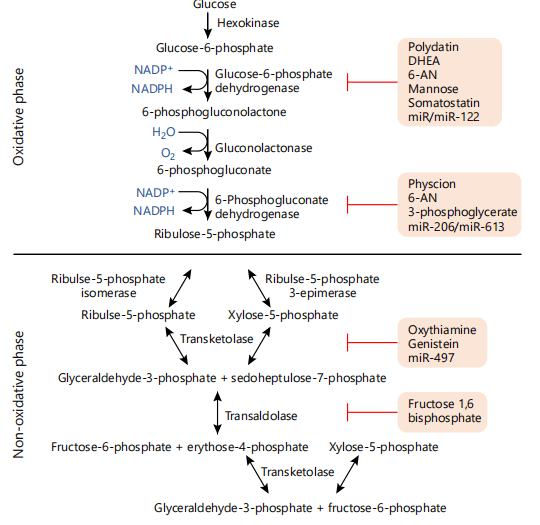
Fig. 1 Schematic representation of the oxidative and nonoxidative phases of the PPP as well as various inhibitors of specific enzymes that may be targeted in cancer treatments as covered in this review.
Cancer cells hijack the PPP for these purposes, relying on its outputs to counteract oxidative stress and meet biosynthetic needs. This metabolic reprogramming allows them to withstand challenging tumor microenvironments, such as hypoxia and nutrient scarcity, where ROS levels are often elevated. Notably, cancer cells often show an upregulation of glucose-6-phosphate dehydrogenase (G6PD), the enzyme catalyzing the rate-limiting step of the PPP’s oxidative phase, underscoring its importance in cancer survival. Consequently, the PPP has emerged as a promising target in cancer therapy, with ongoing research focusing on inhibitors that may impair cancer cell growth by disrupting this pathway.
Structure and Function of the Pentose Phosphate Pathway
The Pentose Phosphate Pathway (PPP) is a vital metabolic route branching from glycolysis, and it plays a crucial role in cellular metabolism, particularly in cancer cells. The PPP consists of two distinct phases: the oxidative phase and the nonoxidative phase. These phases work together to support cancer cell proliferation and survival by producing essential intermediates for biosynthesis and reducing agents to counter oxidative stress.
In the oxidative phase, glucose-6-phosphate dehydrogenase (G6PD) catalyzes the conversion of glucose-6-phosphate to 6-phosphogluconate, leading to the production of NADPH. NADPH is essential for maintaining cellular redox balance by neutralizing reactive oxygen species (ROS) and is a critical reducing agent in lipid and nucleotide synthesis pathways. This antioxidant capacity is particularly important for cancer cells, which often exist in hypoxic, high-stress environments that produce significant ROS. Elevated levels of NADPH are also required for the biosynthetic demands of rapidly dividing cancer cells, as it supplies reducing power for fatty acid synthesis and other anabolic processes.
In the nonoxidative phase, the PPP generates ribose-5-phosphate through the action of transketolase (TKT) and transaldolase (TALDO), which are essential for nucleotide synthesis. Ribose-5-phosphate serves as a precursor for DNA and RNA, facilitating the high levels of cell division characteristic of cancer cells. The reversible nature of the nonoxidative phase allows cancer cells to adaptively shunt intermediates between glycolysis and the PPP based on their metabolic needs.
The PPP is regulated by several oncogenes and tumor suppressors. For example, p53, a well-known tumor suppressor, can inhibit G6PD activity, thereby modulating PPP flux and restricting NADPH production under normal conditions. However, in many cancers, this regulation is disrupted, leading to increased PPP activity that supports tumor growth and survival. Thus, understanding the structure and function of the PPP offers insight into how cancer cells meet their biosynthetic and redox requirements, making the PPP a promising target for cancer therapeutics.
Regulation of the Pentose Phosphate Pathway in Cancer Cells
The regulation of the Pentose Phosphate Pathway (PPP) is essential for cancer cells as it fulfills their heightened biosynthetic and antioxidant needs. Multiple cellular mechanisms, influenced by oncogenes and tumor suppressors, orchestrate the activity of key PPP enzymes, ensuring a steady supply of metabolic intermediates. The PPP’s oxidative phase is particularly crucial because it produces nicotinamide adenine dinucleotide phosphate (NADPH), a molecule that cancer cells use to neutralize reactive oxygen species (ROS) and sustain anabolic processes.
A primary regulator of the PPP is the tumor suppressor protein p53, which can restrict PPP activity by inhibiting glucose-6-phosphate dehydrogenase (G6PD), the first enzyme of the oxidative phase. p53 achieves this inhibition by directly binding to G6PD, preventing its dimerization and enzymatic activation. This action shifts cellular glucose metabolism away from the PPP, limiting NADPH production and consequently restricting cancer cell growth. However, in many cancer types, p53 function is compromised, leading to unrestrained PPP flux and supporting tumor progression.
Conversely, oncogenes like Ras and Myc are known to upregulate the PPP. The Ras signaling pathway enhances glucose uptake and drives glycolysis and PPP activity, partly by upregulating glucose transporter 1 (GLUT1) and hexokinase enzymes, both crucial for glucose metabolism. The Myc oncogene also promotes the PPP by increasing the expression of enzymes like transketolase (TKT), which catalyzes a rate-limiting step in the PPP’s nonoxidative phase. This elevated flux through the PPP facilitates nucleotide production, bolstering cancer cell proliferation and survival.
Other cellular stress responses, such as the activation of AMP-activated protein kinase (AMPK) during nutrient scarcity, further influence PPP regulation. AMPK activation can divert glucose metabolites into alternative pathways to maintain NADPH levels, aiding cancer cells in overcoming oxidative stress. In summary, the interplay between tumor suppressors, oncogenes, and stress-responsive pathways finely tunes the PPP in cancer cells, highlighting it as a strategic therapeutic target for disrupting cancer metabolism.
Targeting the Pentose Phosphate Pathway (PPP) in Cancer Therapy
The Pentose Phosphate Pathway (PPP) has become a promising target in cancer therapy due to its essential role in meeting cancer cells’ metabolic demands. By inhibiting the PPP, researchers aim to reduce the production of NADPH and ribose-5-phosphate, which are crucial for cellular antioxidant defense and nucleotide synthesis, respectively. Targeting these aspects of the PPP could effectively starve cancer cells of the resources needed for rapid proliferation and survival under oxidative stress.
Several inhibitors for the PPP are currently under investigation, particularly those targeting enzymes in the oxidative phase. For example, 6-aminonicotinamide (6-AN) is a competitive inhibitor of G6PD, which blocks NADPH production and induces oxidative stress in cancer cells. However, 6-AN’s clinical application has been limited due to toxicity concerns. Polydatin, a natural compound, has shown promise in preclinical studies by inhibiting G6PD, inducing ROS accumulation, and promoting cancer cell apoptosis with fewer side effects. Another inhibitor, oxythiamine, targets transketolase (TKT) in the nonoxidative phase and has been shown to limit tumor growth by blocking nucleotide synthesis.
Combination therapies also show potential for enhancing PPP inhibitor efficacy. For instance, pairing PPP inhibitors with chemotherapeutic agents like cisplatin has demonstrated synergistic effects, as PPP inhibition sensitizes cancer cells to chemotherapy by disrupting redox balance. Additionally, targeting the PPP may be particularly beneficial in tumors with elevated PPP activity, which are often more resistant to standard treatments.
In sum, targeting the PPP disrupts cancer cells’ metabolic resilience, limiting their ability to grow and survive. While several promising inhibitors exist, further research is essential to develop selective and low-toxicity compounds that can effectively translate PPP inhibition into clinical cancer therapies.
Future Directions and Clinical Implications of Targeting the PPP
Looking ahead, the PPP offers numerous opportunities for innovative cancer therapies, especially in tumors exhibiting high PPP activity. Increased PPP flux is often associated with resistance to conventional treatments, as the pathway supports enhanced biosynthesis and a robust antioxidant defense. Inhibiting the PPP, therefore, holds promise for sensitizing resistant cancers to existing treatments by undermining their metabolic resilience.
Several PPP inhibitors are currently being researched, yet most have not advanced to clinical trials due to toxicity or efficacy concerns. A primary goal in future research is to develop selective PPP inhibitors with minimal off-target effects. Natural compounds, such as polydatin and dehydroepiandrosterone (DHEA), have shown promise as G6PD inhibitors in preclinical studies, exhibiting lower toxicity profiles compared to synthetic inhibitors. Ongoing efforts aim to refine these compounds to enhance specificity and minimize side effects, making them more viable for clinical use.
Combining PPP inhibition with other therapies, particularly chemotherapy and radiation, is another promising approach. For example, studies have shown that co-administration of PPP inhibitors with drugs like cisplatin can enhance cancer cell sensitivity by disrupting their antioxidant capacity and redox homeostasis. This approach may lead to more effective, lower-dose chemotherapy regimens with fewer side effects, benefitting patients with advanced cancers.
In conclusion, the future of PPP-targeted therapies lies in the development of selective inhibitors and combination strategies that can be applied across cancer types. By disrupting a fundamental aspect of cancer cell metabolism, PPP inhibitors could serve as powerful adjuncts to conventional therapies, improving outcomes for patients with difficult-to-treat cancers.
References
- Ghanem, N., El-Baba, C., Araji, K., El-Khoury, R., Usta, J., & Darwiche, N. (2021). The pentose phosphate pathway in cancer: Regulation and therapeutic opportunities. Chemotherapy, 66(5–6), 179–191.
- Liberti, M. V., & Locasale, J. W. (2016). The Warburg effect: How does it benefit cancer cells? Trends in Biochemical Sciences, 41(3), 211-218.
- Pavlova, N. N., & Thompson, C. B. (2016). The emerging hallmarks of cancer metabolism. Cell Metabolism, 23(1), 27-47.
- Patra, K. C., & Hay, N. (2014). The pentose phosphate pathway and cancer. Trends in Biochemical Sciences, 39(8), 347-354.
- Jin, L., & Zhou, Y. (2019). Crucial role of the pentose phosphate pathway in malignant tumors. Oncology Letters, 17(5), 4213–4221.
- Kowalik, M. A., Columbano, A., & Perra, A. (2017). Emerging role of the pentose phosphate pathway in hepatocellular carcinoma. Frontiers in Oncology, 7, 87.
- Lu, S. C. (2013). Glutathione synthesis. Biochimica et Biophysica Acta (BBA)-General Subjects, 1830(5), 3143–3153.
- Stincone, A., Prigione, A., Cramer, T., Wamelink, M. M., Campbell, K., Cheung, E., … & Trezzi, J. P. (2015). The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biological Reviews, 90(3), 927–963.
- Giacomini, I., Ragazzi, E., Pasut, G., & Montopoli, M. (2020). The pentose phosphate pathway and its involvement in cisplatin resistance. International Journal of Molecular Sciences, 21(3), 937.