The Application of Chiral Molecules in Our Lives
Abstract
This article explores the expanding role of chiral molecules in scientific innovation, particularly highlighting their applications in nanotechnology, materials science, and green chemistry. As chirality continues to be a focal point in research, its potential to revolutionize medical diagnostics, targeted drug delivery, and sustainable manufacturing processes is increasingly evident. The integration of chiral molecules into these fields promises enhanced efficacy, reduced environmental impact, and the development of new technologies. This review underscores the importance of continued research and investment in chirality, suggesting that it holds keys to addressing some of the most pressing challenges in medicine, industry, and environmental sustainability.
Introduction to Chiral Molecules
Chirality, a fundamental property in molecular chemistry, plays a critical role across various scientific and industrial fields. The term “chiral” comes from the Greek word for hand, reflecting how chiral molecules, like human hands, exist in two forms that are mirror images but not superimposable. This unique characteristic influences everything from the efficacy of pharmaceutical drugs to the taste of food and the safety of agricultural chemicals.
At its core, a chiral molecule contains an asymmetric carbon center, typically bonded to four different substituents. This structural asymmetry results in two distinct configurations: left-handed (L-) and right-handed (D-), each capable of different interactions at the molecular level. In the pharmaceutical industry, this difference is paramount as the two forms of a drug can have drastically different effects in the human body. For instance, one enantiomer may be therapeutic, while its counterpart could be ineffective or even harmful.
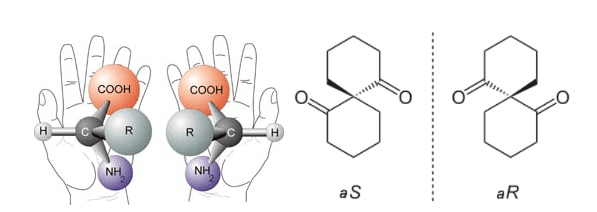
Fig 1. Chiral Molecules
The implications of chirality extend beyond medicine. In agriculture, chiral pesticides ensure that only targeted pests are affected, reducing environmental toxicity and enhancing crop safety. In the food industry, the chirality of molecules can affect flavors and aromas, which are crucial for consumer satisfaction and product success.
Understanding and applying chirality can lead to innovations that enhance the quality of life, reduce adverse effects, and create more sustainable practices across industries. As research and technology advance, the role of chiral molecules continues to expand, making their study a vital component of scientific progress and industrial application.
Definition of chirality in chemistry
Chirality is a geometric property of some molecules characterized by their inability to be superimposed onto their mirror images, similar to how left and right hands are mirror opposites. This fundamental concept in molecular chemistry has profound implications across various scientific domains, particularly in understanding how molecular interactions occur in nature and in synthetic environments.
At the molecular level, chirality occurs due to the presence of an asymmetric carbon atom, typically bonded to four different groups or atoms. This asymmetry creates two versions of the molecule, known as enantiomers. Each enantiomer can exhibit distinct physical, chemical, and biological properties despite having the same molecular formula. The differences between enantiomers can be so significant that they behave almost like completely different substances. For example, the smell of oranges versus lemons is due to the different spatial arrangements of the same molecular formula in limonene.
In nature, chirality is ubiquitous and often critical for biological function. Many biological molecules, such as DNA, proteins, and carbohydrates, are chiral. Their interactions with other molecules, including drugs and nutrients, are highly dependent on their specific orientations. This selective interaction can dictate biological pathways and influence drug design, where the right enantiomer can mean the difference between a cure and a toxin.
The significance of chirality in molecular interactions cannot be overstated. Pharmaceuticals often require the correct enantiomer to effectively bind to specific biological targets. The infamous case of thalidomide, where one enantiomer caused severe birth defects while the other acted as a sedative, dramatically underscores the critical nature of chirality in drug synthesis and usage.
Role of chirality in drug effectiveness and safety
Chirality plays an essential role in pharmaceutical applications, where the specific orientation of molecules can greatly influence both the efficacy and safety of medications. The distinction between different enantiomers of a drug is not merely structural but also pharmacological, as each enantiomer can interact differently with the body. For example, in the case of the drug thalidomide, one enantiomer was effective as a sedative and anti-nausea medication, while its mirror image caused severe birth defects. This tragic outcome underscores the critical importance of enantiomeric purity in drug design and the stringent regulatory standards that now exist to ensure the safety of chiral drugs.
Moreover, developing single-enantiomer drugs, known as enantiopure drugs, has become a focus in pharmaceutical research. These drugs, consisting of only one enantiomer, aim to maximize therapeutic efficacy and minimize side effects. A prominent example is esomeprazole, the S-enantiomer of omeprazole, which is more effective in treating acid reflux with fewer doses compared to its racemic mixture. The enhanced specificity of enantiopure drugs highlights the advances in chiral technology and stereoselective synthesis, which are central to modern pharmaceutical science.
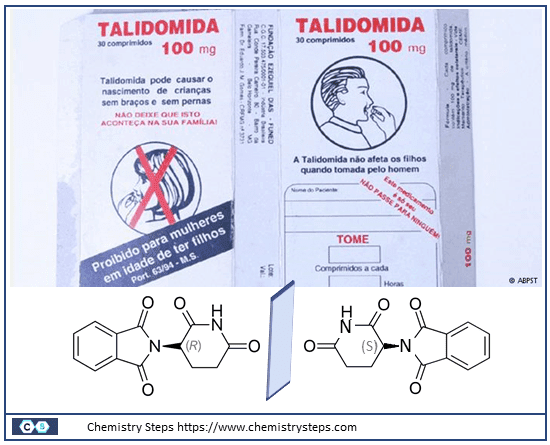
Fig 2. Chemical structure and (+)-(R) and (-)-(S) enantiomers of thalidomide
Transitioning from pharmaceuticals to agriculture, chirality also significantly impacts the effectiveness and environmental footprint of agrochemicals. Chiral pesticides, for instance, are designed to target specific pests more efficiently while reducing harm to non-target organisms and minimizing environmental contamination. The selective nature of such pesticides can lead to more sustainable farming practices by achieving the desired pest control with lower quantities of active substances.
Use of chiral molecules in pesticides and herbicides
The application of chiral molecules in agriculture extends beyond pesticides to include pheromones used in pest management. Chiral pheromones can be synthesized to match the exact configuration used by certain insects, allowing them to be used as lures or repellents in traps that control pest populations without the collateral damage associated with broad-spectrum insecticides.
Both pharmaceutical and agricultural applications of chiral molecules demonstrate the importance of understanding and manipulating the chirality of compounds to achieve desired outcomes, whether they be therapeutic effects or pest control. This dual focus not only enhances efficiency and specificity but also aligns with broader goals of sustainability and reduced environmental impact. As research continues to advance in these areas, the role of chirality is set to become even more pivotal in developing solutions that are both effective and environmentally responsible.
Food and Flavor Industry
Chirality profoundly influences the food and flavor industry, where the sensory qualities of products—taste, smell, and even appearance—can hinge on the molecular orientation of chiral compounds. The difference between the two enantiomers of a chiral molecule can be as distinct as the flavors of caraway seeds and spearmint, both of which derive from the same chemical compound but in different chiral forms. This subtle yet significant distinction underscores the importance of stereochemistry in food science, particularly in flavor synthesis and food additives.
For instance, the chiral molecule limonene presents in two forms: one imparts the smell of oranges, while the other gives lemons their fresh scent. Such nuances are critical in the food industry, which relies on synthetic versions of these molecules to enhance or replicate flavors and aromas in processed foods. The ability to synthesize and utilize specific enantiomers enhances product consistency and quality, meeting consumer expectations and regulatory standards for food safety and labeling.
Moreover, chirality is not only a concern in terms of flavor but also in terms of biological activity. Certain chiral compounds can interact differently with the human body, affecting nutritional value and metabolic processing. This interaction becomes particularly significant in the development of food supplements and nutraceuticals, where the desired health benefits are often linked to specific enantiomers.
Transitioning to environmental considerations, chiral molecules also play a crucial role in understanding and managing the ecological impact of human activities. Many pollutants that affect air, water, and soil quality are chiral, and their behavior in the environment can vary dramatically between enantiomers. For example, the degradation rates and toxicity of chiral pesticides can differ, influencing how they should be managed and regulated to minimize ecological damage.
Chirality in environmental toxicology
The study of chiral environmental contaminants involves assessing the distribution, transformation, and ultimate fate of each enantiomer in ecosystems. This research is vital for developing accurate risk assessments and effective pollution control strategies that consider the specific behaviors and effects of each chiral form. Advanced analytical techniques, such as chiral chromatography, play an essential role in this field, allowing scientists to separate and quantify the concentrations of different enantiomers in environmental samples.
Furthermore, the field of green chemistry promotes the development of synthetic methods that not only produce desired chiral molecules more efficiently but also reduce waste and avoid the use of hazardous substances. This approach is particularly important in the production of chiral chemicals, where traditional synthetic routes often generate equal amounts of both enantiomers, necessitating additional steps to isolate the active form.
In conclusion, the implications of chirality in the food and flavor industry and environmental science highlight the broad and impactful role of molecular orientation in our daily lives and the natural world. By advancing our understanding and application of chiral molecules, we can enhance the sensory qualities of food, improve nutritional benefits, and develop more sustainable environmental practices. The ongoing research and technological innovations in these areas promise to further integrate chirality into strategic developments across industries.
The ongoing exploration of chiral molecules is steering a remarkable wave of innovation across multiple scientific disciplines. As we delve deeper into the molecular world, the potential applications of chirality are expanding, promising significant advancements in areas ranging from nanotechnology to new materials and beyond. This exploration is not just refining existing technologies; it is paving the way for groundbreaking discoveries that could redefine our approach to health, environment, and industry.
Future Trends and Innovations
One of the most promising areas of research in chirality involves the integration of chiral molecules into nanotechnology. The unique properties of chiral molecules, such as their ability to polarize light differently when in different forms, make them ideal candidates for the development of advanced optical materials. These materials can be used in creating more effective and less invasive medical imaging tools, improving diagnostic capabilities without the need for radioactive tracers or contrasting agents.
Additionally, the field of materials science is seeing significant advancements through the study of chirality. Researchers are developing chiral materials that can be used in drug delivery systems to target specific cells or organs in the body. This targeted approach not only enhances the efficacy of treatments but also minimizes side effects, a crucial factor in treating chronic or life-threatening conditions.
Moreover, chirality is becoming increasingly important in the realm of sustainable and green chemistry. The ability to selectively synthesize one enantiomer of a molecule reduces waste and improves the efficiency of chemical processes. This selectivity is essential for manufacturing processes that are less harmful to the environment and more cost-effective, aligning with global sustainability goals.
Conclusion
The implications of chirality extend far beyond the confines of chemical labs and pharmaceutical manufacturing. They touch upon fundamental aspects of human health, environmental safety, and technological innovation. As we continue to unravel the complexities of chiral interactions, the potential for new applications seems almost limitless.
The integration of chiral molecules into everyday technology and medicine is not just a possibility; it is already underway. Continued investment in this field is crucial, as it holds the key to solving some of the most pressing challenges of our time, from developing new therapeutic agents to designing materials that are both effective and environmentally benign.
In conclusion, the study of chiral molecules is more than a niche area of chemistry; it is a dynamic field that stands at the forefront of scientific innovation. As research progresses, it will likely lead to more precise medical treatments, more sustainable industrial processes, and even entirely new technologies that we have yet to imagine. Encouraging curiosity and support for this research is essential for fostering the next generation of scientific breakthroughs.
References
- Liu Y, Wu Z, Armstrong DW, Wolosker H, Zheng Y. Detection and analysis of chiral molecules as disease biomarkers. Nat Rev Chem. 2023 May;7(5):355-373. doi: 10.1038/s41570-023-00476-z. Epub 2023 Mar 20. PMID: 37117811; PMCID: PMC10175202.
- Cai WY, Ding QN, Zhou L, Chen J. Asymmetric Synthesis of Axially Chiral Molecules via Organocatalytic Cycloaddition and Cyclization Reactions. Molecules. 2023 May 24;28(11):4306. doi: 10.3390/molecules28114306. PMID: 37298781; PMCID: PMC10254363.
- Niu X, Zhao R, Yan S, Pang Z, Li H, Yang X, Wang K. Chiral Materials: Progress, Applications, and Prospects. Small. 2023 Sep;19(38):e2303059. doi: 10.1002/smll.202303059. Epub 2023 May 22. PMID: 37217989.
- Salama S, Mostafa HS, Husseiny S, Sebak M. Actinobacteria as Microbial Cell Factories and Biocatalysts in The Synthesis of Chiral Intermediates and Bioactive Molecules; Insights and Applications. Chem Biodivers. 2024 Feb;21(2):e202301205. doi: 10.1002/cbdv.202301205. Epub 2024 Jan 17. PMID: 38155095.




