The Efficacy and Safety of Immune Checkpoint Inhibitors in Treating Melanoma
Abstract
This comprehensive review explores the impact of Immune Checkpoint Inhibitors (ICIs) on advanced melanoma treatment. ICIs enhance T-cell activity against cancer cells by blocking immune checkpoints, leading to enduring immune responses. The study reveals substantial improvements in progression-free and overall survival rates, particularly with combination therapies. However, ICI treatment may trigger chronic immune-related adverse events, emphasizing the need for vigilant, long-term monitoring.
Introduce
Melanoma is a deadly cancer caused by uncontrolled division of melanocytes. It accounts for 80% of skin cancer-related deaths. Approximately 200,000 new cases of melanoma are diagnosed each year. Due to advancements in early diagnosis, targeted therapy, and immunotherapy, the survival rate of melanoma has improved.
Immune checkpoint inhibitors (ICIs) can block the interaction between immune checkpoints in T cells and ligands in tumor cells. Programmed cell death protein 1 (PD-1), lymphocyte activation gene-3 (LAG-3), and cytotoxic T-lymphocyte antigen 4 (CTLA-4) are immune checkpoints that regulate immune responses. Nivolumab and pembrolizumab are PD-1 inhibitors, while ipilimumab is a CTLA-4 inhibitor. ICIs can improve the overall survival (OS) and progression-free survival (PFS) of melanoma patients.
When tumor antigens are presented to the T-cell receptor (TCR) via the major histocompatibility complex (MHC), T cells are activated. Co-stimulatory signals also play a crucial role in T-cell activation. This signal is generated when CD28 on the T cell interacts with the B7 molecule on the antigen-presenting cell (APC). When CTLA-4 binds with B7, T cells become inactivated. Ipilimumab activates T cells by blocking the binding of CTLA-4 with B7. PD-1 inhibitors activate T cells by blocking the interaction between PD-1 on T cells and PD-L1 on cancer cells.
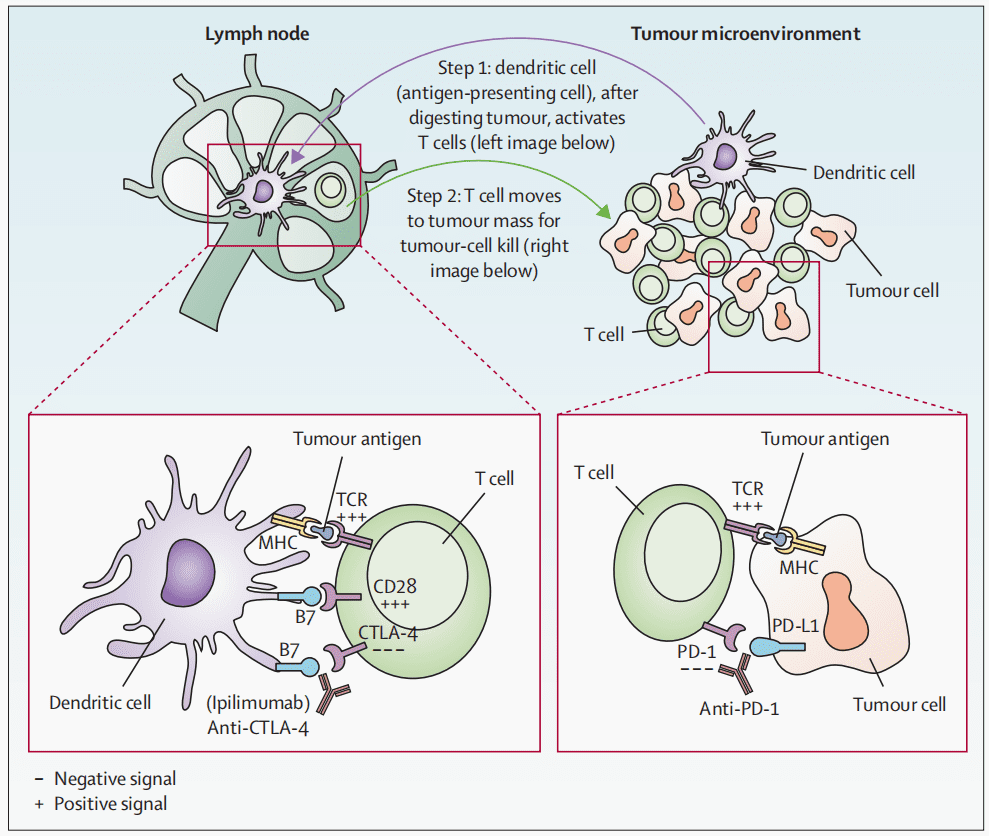
Fig 1. T-cell activation by anti-CTLA4 and anti-PD1 [1].
Significant Survival Benefits of Immune Checkpoint Inhibitors
Immune checkpoint inhibitors can lead to long-term survival in about 50% of advanced melanoma patients. Without immunotherapy, approximately 10% of patients can achieve long-term survival. The five-year overall survival rate with immune checkpoint inhibitors is higher than other melanoma treatment methods. A cohort study involving 16,831 patients showed an improvement in the overall survival of stage IV melanoma patients who received first-line ICI treatment. Patients treated with ICI had a median overall survival of 43.7 months, while those receiving chemotherapy or targeted therapy had a median overall survival of 16.1 months. The median overall survival for PD-1 inhibitors and CTLA-4 inhibitors combined with nivolumab was 33 to 37 months and 72 months, respectively. The five-year overall survival rates for the combination of PD-1 inhibitors and CTLA-4 inhibitors with nivolumab were 45% and 52%, respectively.
How effective is ICI monotherapy?
The effectiveness and safety of PD-1 inhibitors Nivolumab and Pembrolizumab surpass the CTLA-4 inhibitor Ipilimumab. The 5-year overall survival rates for Pembrolizumab and Nivolumab are 43% and 44% respectively, whereas Ipilimumab has a 5-year overall survival rate of 26%. In Phase 3 clinical trial CheckMate 066, melanoma patients without BRAF mutations who received Nivolumab along with the chemotherapy drug Dacarbazine had 1-year overall survival rates of 72.9% and 42.1% respectively.
In the Phase III clinical trial KEYNOTE-006, patients treated with Pembrolizumab and Ipilimumab had median overall survival periods of 32.7 months and 15.9 months, respectively. The median progression-free survival for patients receiving Pembrolizumab and Ipilimumab was 8.4 months and 3.4 months, respectively. The 7-year overall survival rates for patients treated with Pembrolizumab and Ipilimumab were 37.8% and 25.3%, respectively.
The progression-free survival of BRAFV600 mutant melanoma patients who received programmed death ligand 1 (PD-L1) inhibitor atezolizumab combined with two targeted therapeutic drugs Vemurafenib and Cobimetinib was 15.1 months. The progression-free survival of Atezolizumab combined with Cobitinib was similar to that of pembrolizumab. In the phase III clinical trial IMspire170, the median progression-free survival of atezolizumab combined with coubitinib and pembrolizumab monotherapy was 5.5 months and 5.7 months, respectively.
ICI is also effective for melanoma patients at high risk of recurrence. Patients treated with nivolumab and ipilimumab have 4-year recurrence-free survival rates of 51.7% and 41.2%, respectively. Pembrolizumab can also reduce the risk of recurrence. Patients treated with Pembrolizumab have a median recurrence-free survival of 37.2 months.
How effective is combination therapy with ICI?
ICI combined therapy is more effective than single therapy, but the risk of serious adverse reactions is also greater. In this phase 2-3 trial RELATIVITY-047, the combination of Relatlimab and nivolumab significantly improved progression-free survival compared to nivolumab monotherapy. The median progression-free survival of patients treated with Relatlimab combined with nivolumab was 10.1 months, while the median progression-free survival of patients treated with nivolumab was 4.6 months.
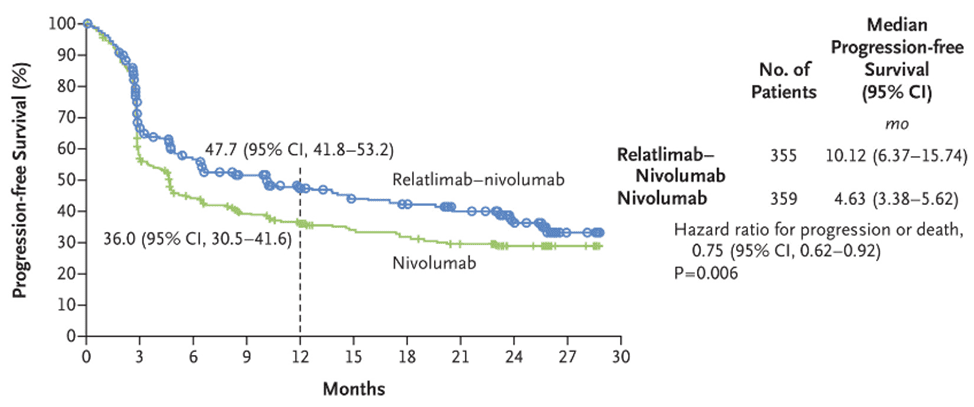
Fig 2. The progression-free survival of patients receiving combined treatment of Relatlimab and Nivolumab, and Nivolumab monotherapy [3].
The combination of Ipilimumab and nivolumab is effective in melanoma patients who do not respond to PD-1 inhibitors. Compared with ipilimumab monotherapy, this combination therapy increased progression-free survival by 37%. The objective response rate (ORR) of patients receiving ipilimumab and nivolumab combination therapy and ipilimumab monotherapy was 28% and 9%, respectively. The 5-year overall survival rate of melanoma patients treated with nivolumab and ipilimumab was 52%. In a phase 2 trial, the combination of monoclonal antibodies Sotigalimab and Nivolumab was safe for metastatic melanoma patients with disease progression after anti-PD-1 treatment. The ORR of patients treated with the combination of these drugs was 15%.
The median progression-free survival of the combined treatment of nivolumab and ipilimumab was significantly higher than that of nivolumab monotherapy or ipilimumab monotherapy. The median progression-free survival of patients receiving nivolumab and ipilimumab combination therapy, nivolumab monotherapy, and ipilimumab monotherapy was 11.5 months, 6.9 months, and 2.9 months, respectively. This combination therapy is also effective for melanoma patients with brain metastasis. The 1-year survival rate of patients with brain metastatic melanoma treated with combined Nivolumab and ipilimumab was 81.5%.
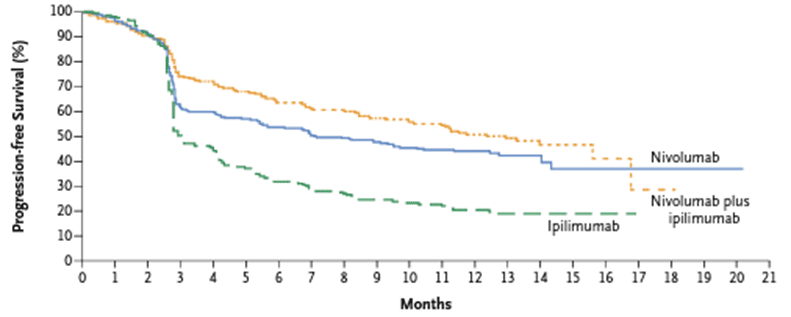
Fig 3. The progression-free survival of patients receiving combined treatment of nivolumab and ipilimumab, and patients receiving nivolumab and ipilimumab separately [4].
In the Phase III clinical trial CheckMate 067, previously untreated melanoma patients who received combined treatment with Nivolumab and Ipilimumab, Nivolumab monotherapy, and Ipilimumab monotherapy had median overall survival rates of 72.1, 36.9, and 19.9 months respectively. For BRAF gene mutated melanoma patients in the same treatment groups, the 6.5-year overall survival rates were 57%, 43%, and 25% respectively.
In the Phase 2 clinical trial CheckMate 069, patients treated with combined therapy of Nivolumab and Ipilimumab, as well as those treated with Ipilimumab monotherapy, had 2-year overall survival rates of 63.8% and 53.6% respectively.
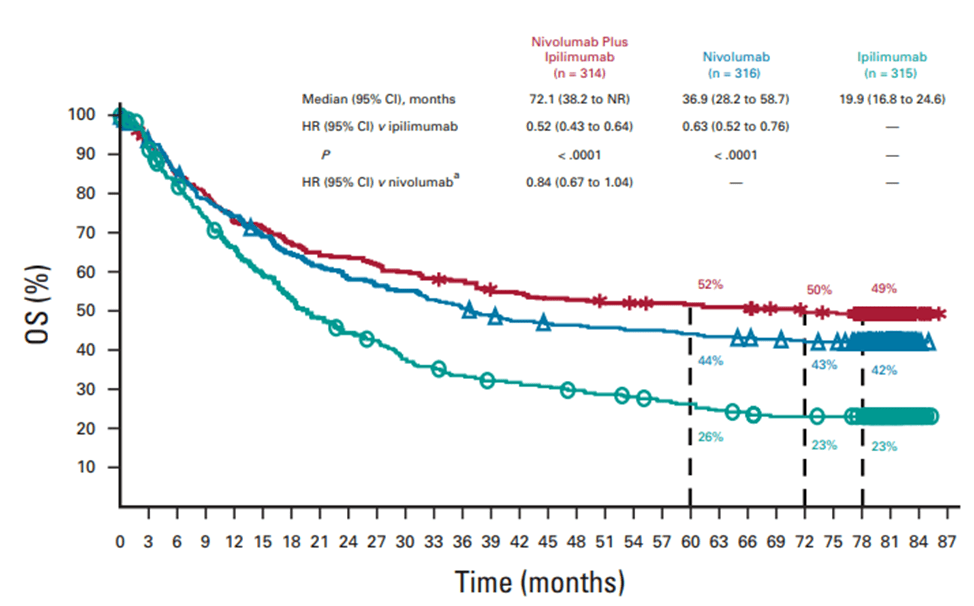
Fig 4. The overall survival (OS) of patients receiving combined treatment of nivolumab and ipilimumab, and patients receiving nivolumab and ipilimumab separately [5].
In a pooled analysis of Phase III clinical trials CheckMate 066 and 067, along with Phase II clinical trial CheckMate 069, 23% of patients receiving combined treatment with Nivolumab and Ipilimumab achieved complete remission, while 19% of patients receiving Nivolumab monotherapy achieved complete remission. Patients who achieved complete remission after receiving combined treatment with Nivolumab and Ipilimumab, as well as those who received Nivolumab monotherapy, had 5-year overall survival rates of 85% and 86% respectively.
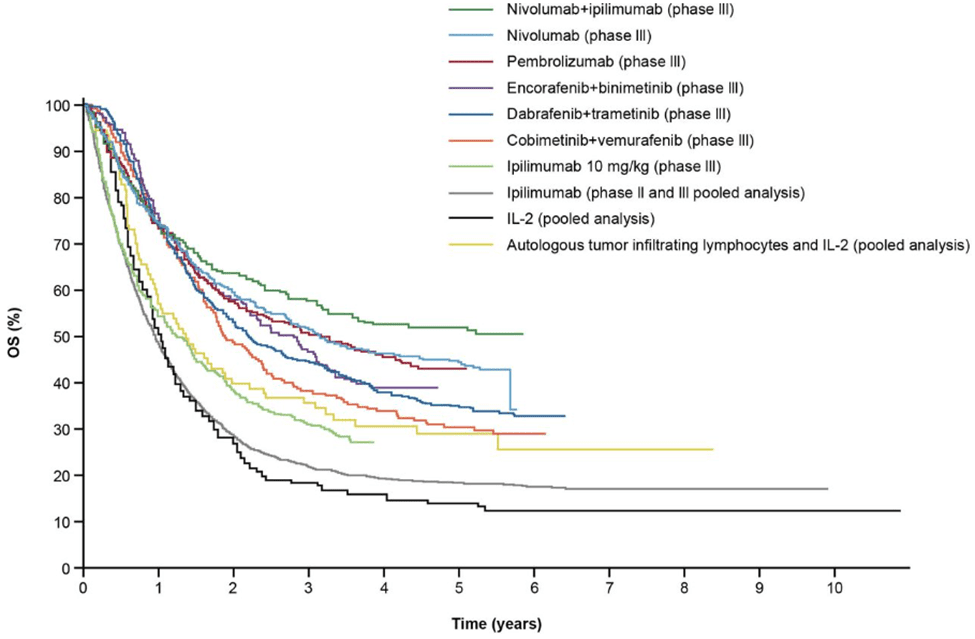
Fig 5. The overall survival of melanoma patients receiving ICI and targeted therapy [6].
What are the side effects of ICI treatment?
When ICI activates T cells, it can lead to immune-related adverse events (irAE) in multiple organs such as the liver and colon. Among 318 patients treated in Australia and the United States, 63.7% and 46.2% experienced acute and chronic irAE respectively. Adverse reactions appeared within the first week of treatment, with toxicity peaking between 6 to 12 weeks. IrAE can affect any organ system, and its effects may be long-lasting. There is a risk of long-term or permanent toxicity, necessitating prolonged use of corticosteroids or other medications for treatment. In a retrospective review of 161 melanoma patients receiving ICI, 41.0% of patients observed permanent irAE, and 9.3% experienced long-term irAE.
ICI can lead to adverse reactions such as hypophysitis, colitis, diabetes, arthritis, pneumonia, and hepatitis. Melanoma patients receiving ICI commonly experience viral skin conditions like lichenoid dermatitis, vitiligo, bullous pemphigoid, and maculopapular rash. Patients treated with the combination of Nivolumab and Ipilimumab may experience severe adverse reactions including elevated alanine aminotransferase levels and colitis. Grade 3-4 irAE induced by ICI include myelitis, adrenal insufficiency, and myocarditis. Adverse reactions from the combination therapy of Atezolizumab and Cobimetinib include diarrhea, hypertension, and elevated creatine kinase levels.
Summary
In summary, ICI enables T cells to target and destroy cancer cells by blocking immune checkpoints. ICI can generate a persistent immune response against cancer cells and significantly improve both progression-free survival and overall survival in advanced melanoma patients. ICI can prolong the recurrence-free survival (RFS) of melanoma patients and enhance the survival rates of those with brain metastases. Melanoma patients receiving ICI treatment may experience chronic immune-related adverse events, necessitating long-term monitoring.
Reference
- Carlino, M. S., Larkin, J., & Long, G. V. (2021). Immune checkpoint inhibitors in melanoma. Lancet (London, England), 398(10304), 1002–1014.
- Lamba, N., Ott, P. A., & Iorgulescu, J. B. (2022). Use of First-Line Immune Checkpoint Inhibitors and Association With Overall Survival Among Patients With Metastatic Melanoma in the Anti-PD-1 Era. JAMA network open, 5(8), e2225459.
- Boutros, A., Tanda, E. T., Croce, E., Catalano, F., Ceppi, M., Bruzzone, M., Cecchi, F., Arecco, L., Fraguglia, M., Pronzato, P., Genova, C., Del Mastro, L., Lambertini, M., & Spagnolo, F. (2023). Activity and safety of first-line treatments for advanced melanoma: A network meta-analysis. European journal of cancer (Oxford, England: 1990), 188, 64–79.
- Larkin, J., Chiarion-Sileni, V., Gonzalez, R., Grob, J. J., Cowey, C. L., Lao, C. D., Schadendorf, D., Dummer, R., Smylie, M., Rutkowski, P., Ferrucci, P. F., Hill, A., Wagstaff, J., Carlino, M. S., Haanen, J. B., Maio, M., Marquez-Rodas, I., McArthur, G. A., Ascierto, P. A., Long, G. V., … Wolchok, J. D. (2015). Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine, 373(1), 23–34.
- Wolchok, J. D., Chiarion-Sileni, V., Gonzalez, R., Grob, J. J., Rutkowski, P., Lao, C. D., Cowey, C. L., Schadendorf, D., Wagstaff, J., Dummer, R., Ferrucci, P. F., Smylie, M., Butler, M. O., Hill, A., Márquez-Rodas, I., Haanen, J. B. A. G., Guidoboni, M., Maio, M., Schöffski, P., Carlino, M. S., … Hodi, F. S. (2022). Long-Term Outcomes With Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients With Advanced Melanoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 40(2), 127–137.
- Michielin, O., Lalani, A. K., Robert, C., Sharma, P., & Peters, S. (2022). Defining unique clinical hallmarks for immune checkpoint inhibitor-based therapies. Journal for immunotherapy of cancer, 10(1), e003024.




