The Multifaceted Role of the Aryl Hydrocarbon Receptor (AhR): From Detoxification to Cancer Therapy
Abstract
The aryl hydrocarbon receptor (AhR) is a transcription factor that governs various functions upon activation by a wide range of ligands, including xenobiotics, natural compounds, microbiome metabolites, and endogenous molecules. Due to its interaction with such diverse ligands, AhR is considered an exposome receptor. A key role of AhR is to regulate multiple defense mechanisms against chemical toxins and bacterial infections. Beyond its well-known detoxification role, AhR is involved in maintaining physiological barriers and plays a vital role in immune system modulation. Additionally, AhR contributes to the development and homeostasis of various organs. Its activity is influenced by the type of ligand and the duration of activation, which can be either sustained or transient, sometimes resulting in opposite regulatory outcomes, such as in the regulation of cancer pathways. Research on selective AhR modulators and their pharmacological properties remains a crucial field of study.
Introduction
Living organisms possess a sophisticated detoxification system that allows them to resist and survive exposure to harmful substances, known as xenobiotics. Extensive research has been conducted to unravel the complexities of this detoxification system, leading to the discovery and characterization of its core components. One of the first major breakthroughs in this field occurred in the late 1950s with the identification of enzymes involved in xenobiotic metabolism. These enzymes were later classified as hemoproteins and named cytochrome P450 (CYP450), which are responsible for the oxidation of various exogenous compounds.
The discovery of the aryl hydrocarbon receptor (AhR) in 1976 by Poland and colleagues marked another significant milestone. They proposed the existence of a receptor that could regulate the induction of enzymes with aryl-hydroxylase activity. AhR, a basic helix-loop-helix/period aryl hydrocarbon receptor nuclear translocator (ARNT) single-minded (bHLH/PAS) transcription factor, plays a critical role in the detoxification system. In its inactive state, AhR is sequestered in the cytoplasm as part of a complex involving multiple partners, including two heat shock protein 90 (Hsp90) chaperones, X-associated protein 2 (XAP2), also known as AhR-interacting protein (AIP), the cellular-sarcoma (c-Src) kinase, and the cochaperone p23.
Upon binding of a ligand to AhR, the receptor undergoes a conformational change, releasing one Hsp90 and exposing its nuclear localization signal (NLS), which allows it to translocate into the nucleus. Once in the nucleus, AhR dissociates from its remaining cytoplasmic partners and forms a heterodimer with its nuclear partner ARNT. This heterodimer can then bind to specific xenobiotic response elements (XREs) in the regulatory sequences of target genes. These XREs have a consensus sequence of 5′-TNGCGTG-3′, with AhR recognizing the TNGC half-site and ARNT recognizing the GTG half-site. The binding of the AhR/ARNT complex to these elements initiates the transcription of genes involved in detoxification, including cytochrome P450 enzymes that metabolize xenobiotics into less harmful metabolites that can be eliminated from the body.
The AhR system is particularly responsive to synthetic exogenous ligands, such as halogenated aromatic hydrocarbons (HAHs) and polycyclic aromatic hydrocarbons (PAHs). HAHs, including the well-known toxic compound 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), and PAHs such as 3-methylcholanthrene and benzo(a)pyrene, have a high affinity for AhR, with dissociation constants (KDs) ranging from pico- to nanomolars for HAHs and from nano- to micromolars for PAHs. These pollutants are notorious for their toxic effects, including skin damage like chloracne from acute exposure to dioxins and long-term health issues such as developmental abnormalities, reproductive issues, immunotoxicity, and thyroid disruption.
In addition to its well-characterized genomic pathway, AhR also participates in nongenomic signaling pathways. Upon ligand binding, AhR interacts with a variety of proteins, including Maf, Kruppel-like factor 6 (KLF6), retinoblastoma protein (RB), and Cullin 4B (CUL4B), which is an E3-ubiquitin ligase. These interactions trigger a cascade of cellular events. For example, exposure to TCDD in rats leads to a rapid increase in intracellular calcium (Ca2+) levels, which activates protein kinase C (PKC). PKC, in turn, phosphorylates cytosolic phospholipase A2 (cPLA2), resulting in the release of arachidonic acid, which is further metabolized into pro-inflammatory molecules like prostaglandins. Furthermore, the release of c-Src from the cytoplasmic AhR complex activates mitogen-activated protein (MAP) kinases, which promote the transcription of cyclooxygenase 2 (COX2), an enzyme that plays a critical role in inflammation.
The activation of AhR also influences other critical cellular pathways, including those involving nuclear factor-kappa B (NF-κB), estrogen receptor (ER)α, and β-catenin. These interactions have broad implications for cellular processes such as proliferation, differentiation, inflammation, and even endocrine disruption. For example, c-Src activation can modulate focal adhesion kinase (FAK) activity, thereby affecting cellular adhesion and migration. Such intricate signaling networks underscore the importance of AhR in regulating not only detoxification but also broader physiological functions.
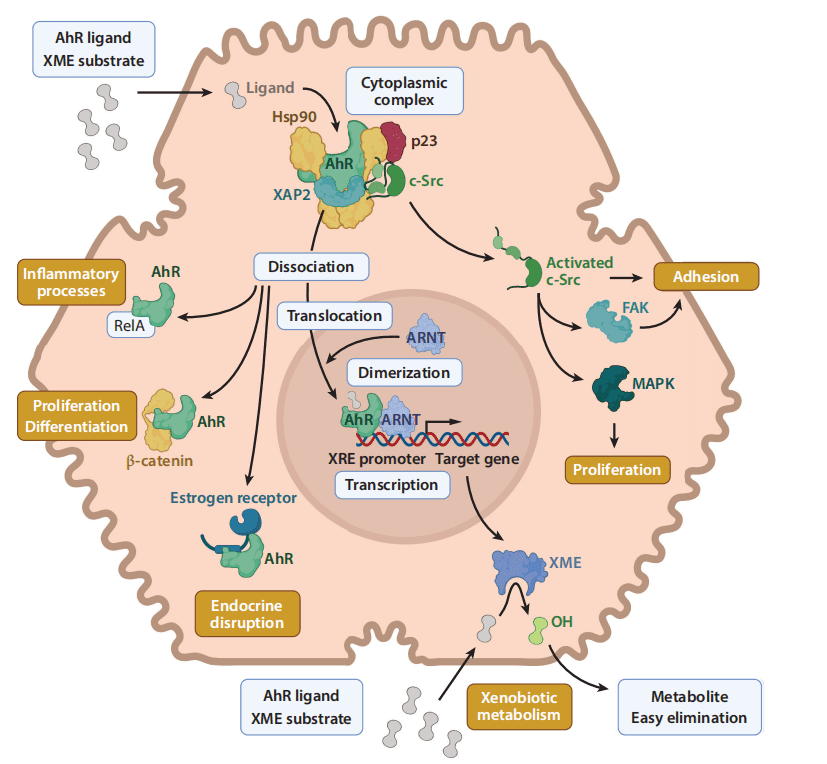
Figure 1. AhR signaling pathways.
The AhR signaling pathway is thus highly complex, involving both genomic and nongenomic mechanisms that regulate a wide range of biological processes. Its role extends beyond detoxification, influencing cellular proliferation, inflammation, and even cancer pathways. The ability of AhR to interact with various signaling molecules highlights its versatility and importance in maintaining cellular homeostasis in the face of environmental stressors.
THE CASE OF AhR FUNCTIONS IN CANCER
A significant body of research has linked the aryl hydrocarbon receptor (AhR) to cancer, fueling a controversial debate on whether the receptor plays a protumorigenic or antitumorigenic role. Many studies suggest that AhR has a protumorigenic function, as it is often overexpressed in cancerous tissues compared to noninvasive tissues. This overexpression has been observed in a range of cancers, including breast, leukemia, lung, liver, stomach, head and neck, cervix, and ovarian cancers. However, only a few studies have investigated how AhR contributes to cancer progression in nonmalignant cells. In human benign mammary cells, for example, Brooks and Eltom found that high levels of AhR were associated with a modified cell cycle and increased cell migration, suggesting that AhR might induce malignant transformations in normal cells.
In vivo studies have similarly provided insights into the tumor-promoting effects of AhR. For instance, Moennikes et al. found that mice with constitutively active AhR developed liver tumors at a rate of 55% compared to just 6% in wild-type mice. Similarly, Currier et al. reported that 75% of mice exposed to 7,12-dimethylbenz[a]anthracene (DMBA) developed mammary tumors, with increased AhR expression levels observed in the tumors. Additionally, high AhR expression has been correlated with the stage of disease progression, from normal to precancerous and cancerous stages. Clinical studies have shown that elevated AhR levels are associated with factors such as tumor stage, histological grade, lymph node involvement, and survival outcomes in cancers such as clear cell carcinoma, urothelial cancer, and hepatocellular carcinoma. Moreover, in aggressive Her2+ breast cancer, high AhR levels have been linked to Her2+ expression, further emphasizing its potential role in tumor progression.
The localization of AhR is also critical. Normally, AhR remains in the cytoplasm until it is activated by a ligand, at which point it translocates into the nucleus. However, constitutive nuclear localization of AhR has been observed in some cancer types and is associated with a worse prognosis. The AhR pathway has been implicated in prometastatic processes such as resistance to apoptosis, invasiveness, altered cell cycle progression, and increased cell migration and proliferation. Studies using knockdown approaches and inhibitors of AhR have further elucidated these mechanisms in breast, renal, lung, head and neck, and urothelial cancers.
A particularly important process regulated by AhR is epithelial-mesenchymal transition (EMT), a key event in cancer metastasis. Several studies have shown that AhR activation increases the expression of EMT markers like Slug and E-cadherin, which also promote stem cell-like properties in cancer cells. For example, Stanford et al. studied triple-negative inflammatory breast cancer and found that overexpression of AhR led to the development of cancer stem-like cells.
The role of the tumor microenvironment in cancer progression is gaining increasing attention, and AhR is believed to play a significant role in this context. High levels of AhR in tumor-infiltrating lymphocytes have been associated with worse outcomes in clear cell carcinoma. AhR also promotes a proinflammatory tumor environment through the secretion of interleukins such as IL-6 and IL-22. In vivo studies have demonstrated that AhR agonists can increase active nuclear AhR not only in tumors but also in surrounding stromal cells, suggesting that AhR contributes to the overall tumor microenvironment. Moreover, experiments have shown that AhR+ fibroblasts can induce larger and more aggressive tumors in mice compared to AhR-deficient fibroblasts.
AhR has also been implicated in chemotherapy resistance. For example, in triple-negative breast cancer, knockout of AhR resulted in increased radiation-induced apoptosis and an enhanced response to paclitaxel, a chemotherapeutic agent. Genes involved in drug resistance, such as ABCC3, were found to be downregulated in AhR-knockout cells, suggesting that targeting AhR could improve chemotherapy effectiveness. In vivo, AhR overexpression has been associated with increased metastasis, while its knockout reduced metastasis in breast cancer models.
Despite the evidence supporting the protumorigenic role of AhR, some studies suggest that the receptor may also have antitumorigenic effects. For instance, certain studies have found no correlation between AhR expression and tumor aggressiveness in various cancers. Additionally, AhR activation with exogenous agonists has been shown to reduce tumor invasiveness, proliferation, and migration in breast and pancreatic cancers. Overactivation of AhR with tranilast, for example, was found to be more effective than standard chemotherapy drugs in reducing the number of mammospheres in breast cancer. Furthermore, AhR activation has been shown to reduce cancer stem cell properties by decreasing markers like ALDH, Notch1, and β-catenin.
AhR also has anti-inflammatory effects within the tumor microenvironment. For example, tranilast has been shown to inhibit the production of proinflammatory cytokines such as IL-6 and IL-17, creating a less favorable environment for tumor growth. In addition, AhR can modulate endocrine signaling in breast cancer, where it has been found to suppress estrogen receptor activity, leading to an antiestrogenic effect. AhR agonists have been shown to reduce estrogen-dependent gene expression and tumor growth, suggesting potential for combination therapies that utilize both AhR agonists and standard treatments like tamoxifen.
The discrepancies in AhR’s role in cancer may be due to various factors, including the type of cancer, the source of AhR activation (endogenous or exogenous ligands), and the nature of the ligand (transient or sustained activation). Experimental data also show variability depending on the models used and the specific ligands involved. For example, endogenous ligands generally promote tumor growth, whereas exogenous ligands may have antiproliferative effects.
In conclusion, AhR represents a promising target for cancer therapy, particularly for cancers that are difficult to treat, such as triple-negative breast cancer. The development of selective AhR modulators (SAhRMs) could allow for more targeted treatments that take into account tissue specificity and patient variability. Personalized medicine approaches that consider individual AhR expression levels and responses to treatment could further enhance the therapeutic potential of targeting the AhR pathway in cancer.
CONCLUSION
The discovery of the adaptive AhR–xenobiotic metabolizing enzyme (XME) pathway in the 1990s initially reinforced the belief that the primary role of the aryl hydrocarbon receptor (AhR) was in xenobiotic metabolism. However, with the advent of knockout (KO) models and omics technologies in the early 21st century, new hypotheses emerged suggesting that AhR could regulate various physiological processes and bind to endogenous ligands. Since then, multiple additional signaling pathways (such as NF-κB, ER, Wnt/β-catenin, c-Src, FAK, and HIF-1α), along with a wide array of ligands (xenobiotics, natural products, endogenous molecules, and microbiota-derived compounds), have been linked to AhR function.
AhR’s regulatory effects are now understood to depend on the mode of exposure and ligand persistence (acute vs. chronic activation, persistent vs. nonpersistent). For example, both AhR deficiency and chronic AhR activation can lead to similar conditions, such as liver steatosis or fibrosis, as seen in AhR KO mice and those treated with kynurenine (KYN) or TCDD.
Selective AhR modulators (SAhRMs) are being explored to treat conditions related to AhR dysregulation. Another therapeutic strategy involves modulating ligand concentrations, with clinical trials targeting the inhibition of KYN synthesis, though this poses potential side effects. Probiotics, such as Lactobacillus reuteri, which produce AhR ligands, are also being studied to counteract inflammation.
Several AhR ligands have shown potential in treating experimental arthritis, and some, such as tapinarof, are in clinical trials for psoriasis and atopic dermatitis. Other promising molecules include tetrandrine, norisoboldine, berberine, indole-3-carbinol, resveratrol, curcumin, and coal tar, with each showing distinct immunomodulatory effects. These molecules, despite their low oral bioavailability, are being considered for the treatment of immune disorders such as colitis, lupus, multiple sclerosis, type 1 diabetes, and even viral infections like Zika and COVID-19.
Reference
- Annu. Rev. Pharmacol. Toxicol. 2022. 62:383–404 First published as a Review in Advance on September 9, 2021 The Annual Review of Pharmacology and Toxicology
- Cooper, D. Y., Levin, S., Narasimhulu, S., & Rosenthal, O. (1965). Photochemical action spectrum of the terminal oxidase of mixed function oxidase systems. Science, 147(3656), 400–402.
- Ohtake, F., Baba, A., Takada, I., Okada, M., Iwasaki, K., et al. (2007). Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature, 446(7135), 562–566.
- Abbott, B. D., Birnbaum, L. S., & Perdew, G. H. (1995). Developmental expression of two members of a new class of transcription factors: I. Expression of aryl hydrocarbon receptor in the C57BL/6N mouse embryo. Developmental Dynamics, 204(2), 133–143.
- Ishida, M., Mikami, S., Shinojima, T., Kosaka, T., Mizuno, R., et al. (2015). Activation of aryl hydrocarbon receptor promotes invasion of clear cell renal cell carcinoma and is associated with poor prognosis and cigarette smoke. International Journal of Cancer, 137(2), 299–310.
- Novikov, O., Wang, Z., Stanford, E. A., Parks, A. J., Ramirez-Cardenas, A., et al. (2016). An aryl hydrocarbon receptor-mediated amplification loop that enforces cell migration in ER-/PR-/Her2- human breast cancer cells. Molecular Pharmacology, 90(5), 674–688.
- Jin, U.-H., Lee, S.-O., Pfent, C., & Safe, S. (2014). The aryl hydrocarbon receptor ligand omeprazole inhibits breast cancer cell invasion and metastasis. BMC Cancer, 14, 498.
- Safe, S., Lee, S.-O., & Jin, U.-H. (2013). Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target. Toxicological Sciences, 135(1), 1–16.