The rise of covalent drugs: How to discover potential covalent drugs
Abstract
In recent years, small molecule covalent drugs have begun to emerge in the field of drug research. In this blog, let’s talk about the research progress of covalent compounds in the field of drug research.
Definition and mechanism of small molecule covalent drugs
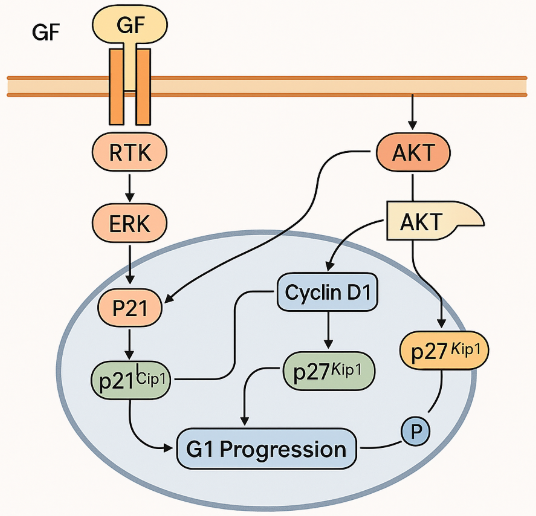
Small molecule covalent drugs are a class of chemical molecules that irreversibly bind to target proteins through covalent bonds to exert biological functions. So how do small molecule covalent drugs work?
First, they reversibly bind to the target enzyme, bringing the electron-rich chemical warhead in the small molecule into close proximity with the active residue in the target; in the second step, a covalent bond is formed between the two reactive entities of the covalent drug and the target.
Common covalent targets include acrylamide, epoxy, chloroacetyl, oxygen sulfonyl fluoride and sulfonyl fluoride, etc. Common amino acid active residues include cysteine, serine, tyrosine, lysine, arginine and glutamate, etc.

Fig.1 The concept of covalent compounds
Characteristics of Covalent Drugs
Compared with non-covalent drugs, what pharmacological advantages do most covalent drugs have?
- Strong and long-lasting effects;
- Low dosage and frequency of administration;
- Pharmacodynamics and pharmacokinetics are separated, and the drug can maintain its efficacy even when it is rapidly cleared;
- Can prevent the development of drug resistance;
- Can target rare, non-conserved residues of specific proteins to achieve higher selectivity, etc.
- But it cannot be ignored that covalent drugs also have disadvantages such as side effects caused by off-target.
Although pharmacologists have a lot of prejudice against drugs that work with covalent bonds, many drugs currently on the market work by covalent bonding. So far, covalent drugs have been discovered either by accident, based on mechanism, or both.
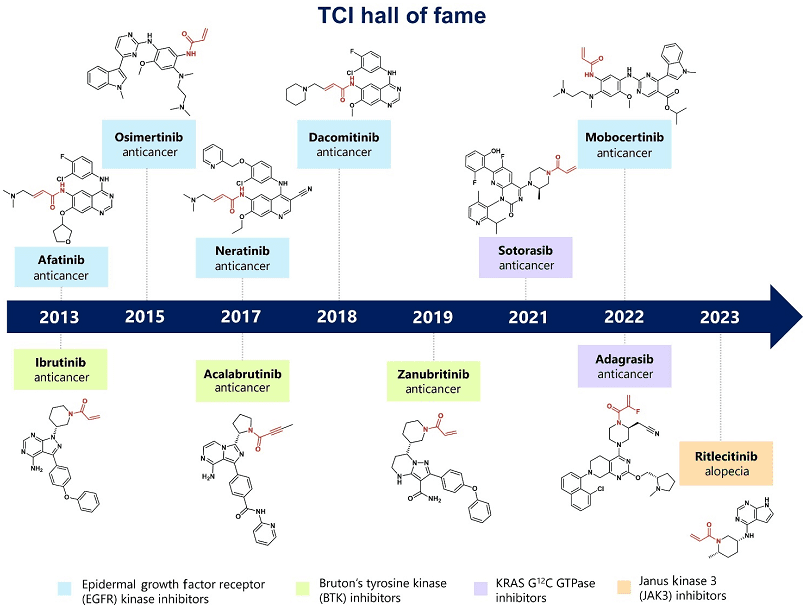
Fig.2 Important drug representatives in the history of covalent drug development (the red structure is the electrophilic warhead)
At the current stage, advances in basic science and technology for drug discovery have facilitated this transformation. For example, proteomics can improve the selectivity of covalent drug targets by determining their binding sites. Due to the advantages of covalent drugs and their clinical effectiveness, the demand for covalent small molecules in the fields of medicinal chemistry and chemical biology is increasing, and the development of methods for rationally designing covalent drugs is particularly critical.
How can we efficiently identify compounds with covalent drug potential?
The selectivity and toxicity of covalent drugs are mainly determined by three aspects: the electrophilicity of the warhead, the nucleophilicity of the target residue, and the non-covalent interaction between the drug ligand and the target.
In terms of the selectivity of covalent inhibitors, the electrophilicity of the warhead plays an extremely important role. Highly reactive electrophilic reagents usually show lower selectivity because they are also prone to attack other parts of the cell in addition to the target of interest. This may lead to the formation of toxic products or adducts, which can cause toxic side effects. Given that excessive electrophilicity may lead to off-target effects, people usually prefer warheads with lower reactivity. After the initial non-covalent binding to the target, the covalent reaction of the target should only proceed with the nucleophilic groups of the target of interest, thereby reducing off-target effects and toxicity. For example, in one study, scientists found that replacing acrylamide warheads with tert-butyl fumarate reduced the off-target rate by 70%. The larger steric volume of the tert-butyl fumarate warhead hinders other side reactions, but maintains the reactivity of the original warhead. Non-covalent interactions with the target also confer inhibitor selectivity. To achieve high selectivity, the non-covalent binding should be strong enough to selectively bind to its target and achieve a high residence time.
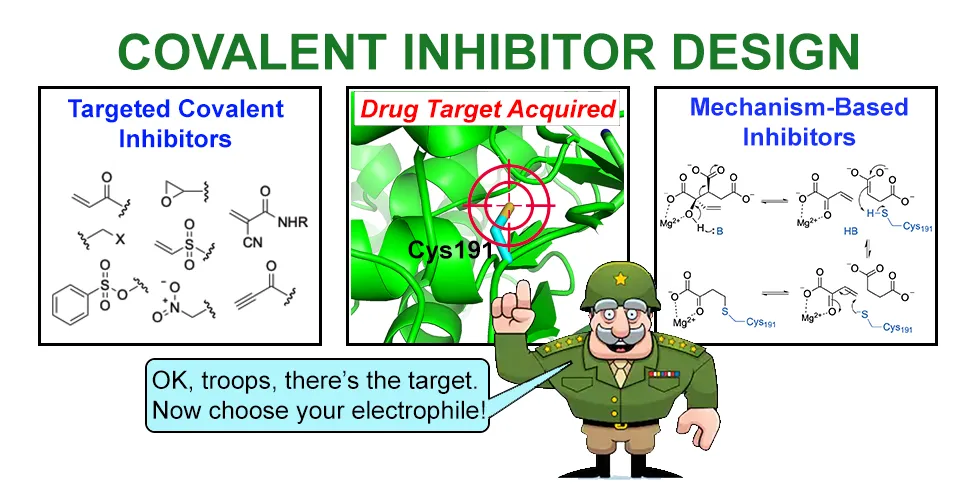
Fig.3 Covalent inhibitor design
Future development direction of covalent small molecule drugs
To date, covalent compounds have been narrowly investigated, and approved covalent drugs have been focused on kinase families and a single cysteine
cluster. Future areas of innovation are in exploring warheads beyond cysteine. Lysine seems most likely to be the next amino acid to be targeted by covalent drugs.
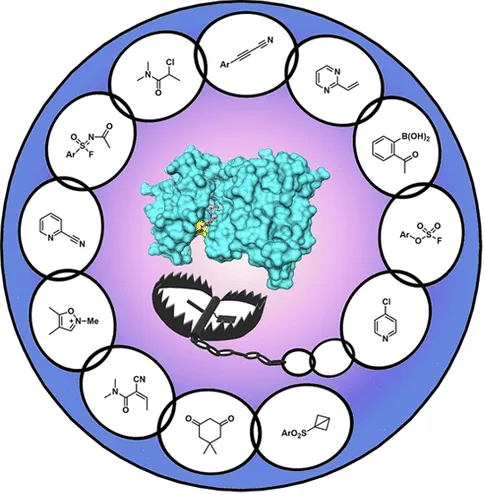
Fig.4 Overview of Targeted Covalent Inhibition Research
Lysine is a more prevalent amino acid in protein binding sites than cysteine and, unlike cysteine, is not restricted to intracellular targets. Cysteine exists intracellularly as a reduced free thiol that can be targeted by covalent inhibitors, whereas it is typically involved in disulfide bonds in extracellular proteins.
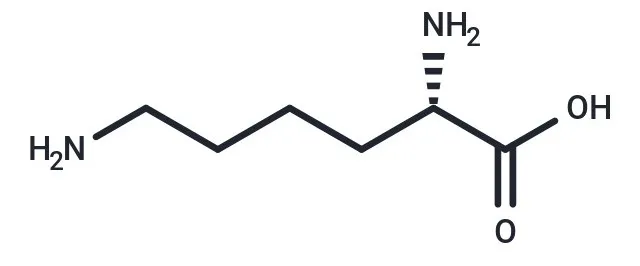
Fig.5 Lysine structure
However, lysine is less nucleophilic than cysteine because it has a higher pKa, which depends on the local protein environment. Taken together, lysine will be more challenging to target with covalent drugs.
Product support
MuseChem can help you discover small molecules of new potential covalent drugs. We have prepared more than 20 types of rich and diverse compound libraries. Mainly including covalent inhibitor preformed compound library, natural product covalent compound library, covalent fragment and other functionally diverse covalent binding compound libraries.
I believe that with the continuous development and improvement of more covalent drug research and development strategies, the screening and use of covalent compounds will usher in vigorous development and bring new hope for the treatment of human diseases.
References
- Lagoutte, R., Patouret, R., & Winssinger, N. (2017). Covalent inhibitors: an opportunity for rational target selectivity. Current Opinion in Chemical Biology, 39, 54–63.
- Mehta, N. V., & Degani, M. S. (2023). The expanding repertoire of covalent warheads for drug discovery. Drug Discovery Today, 28, 103799.
- Ray, S., & Murkin, A. S. (2019). New electrophiles and strategies for mechanism-based and targeted covalent inhibitor design. Biochemistry, 58(52), 5234-5244.
- Gehringer, M., & Laufer, S. A. (2019). Emerging and re-emerging warheads for targeted covalent inhibitors: Applications in medicinal chemistry and chemical biology. Journal of Medicinal Chemistry, 62(12), 5673–5724.