The Role of Cyclin-Dependent Kinases (CDKs) in Cancer: Biomarkers, Therapeutic Targets, and Future Directions
Abstract
Cyclin-dependent kinases (CDKs) play a crucial role in regulating the cell cycle and are central to cancer development due to their frequent dysregulation. CDKs have become significant biomarkers for cancer prognosis, helping predict disease progression and response to treatments. The success of CDK inhibitors, particularly those targeting CDK4/6, has revolutionized cancer therapy, especially for hormone receptor-positive breast cancer. However, resistance to CDK inhibitors remains a challenge, driving the need for further research and the development of next-generation inhibitors targeting other CDKs like CDK7 and CDK9. The future of CDK-targeted cancer therapy lies in personalized treatment strategies, optimized through a deeper understanding of CDK roles in various cancers and through combination therapies that enhance effectiveness and overcome drug resistance.
Introduction to Cyclin Dependent Kinases (CDKs) and Cancer
Cyclin-dependent kinases (CDKs) are a group of serine/threonine kinases with crucial roles in the regulation of cell cycle progression. Te activity of these kinases is induced by cyclins. In fact, CDK/cyclin complexes control progression of the cell cycle in an orderly manner . Emerging evidence suggest that CDKs and cyclins actively participate in the regulation of transcription, epigenetic mechanisms, metabolic processes and self-renewal capacity of stem cells. Most notably,
some of these functions are exerted in an independent manner from establishment of CDKs/cyclins complexes . Another group of proteins, namely cyclin-dependent kinase inhibitors (CKIs) has been revealed to negatively regulate cyclin/CDKs. Te main function of CDKIs is to obstruct cell cycle transition and suppress cell proliferation through inhibition of the enzymatic activity of CDKs. Inhibitor of CDK4 proteins and CDK-interacting protein/kinase inhibitory proteins belong to this
group . Defects in the regulation of cell cycle and mutations in the genes coding cell-cycle regulatory proteins result in unrestrained proliferation of cells leading to formation of tumors. Accordingly, modulation of activity of these proteins by therapeutic agents has been suggested as a promising strategy for treatment of cancers.
Successful introduction of these modalities into clinical settings needs proper recognition of the role of CDKs in the progression of each type of cancer, their interacting molecules and signaling pathways and the efects of suppression of these kinases on malignant features.
In recent years, CDKs have become promising targets for cancer therapies. CDK inhibitors, such as palbociclib, ribociclib, and abemaciclib, have been developed and are already being used clinically to treat certain types of cancer, including breast cancer. These drugs work by blocking the activity of specific CDKs, particularly CDK4 and CDK6, which are often overactive in cancer cells. By inhibiting these kinases, these treatments can slow down the proliferation of cancer cells, induce cell cycle arrest, and, in some cases, trigger cell death.
While CDK inhibitors have shown significant promise, their application is not without challenges. Cancer cells can develop resistance to these drugs, and not all cancers are equally responsive to CDK inhibition. This variability in treatment response highlights the need for further research to better understand the mechanisms underlying CDK dysregulation in different types of cancers and to develop more effective, personalized therapeutic strategies.
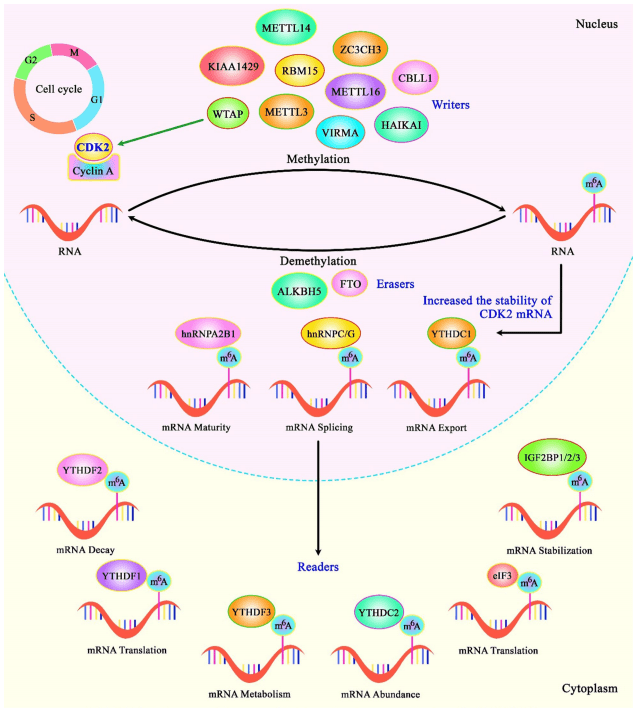
Fig. 1 A schematic diagram of CDK1 and the role of WTAP in modulating CDK2 in renal cell carcinoma.
In summary, CDKs play a central role in the regulation of the cell cycle and are integral to normal cell function. Their dysregulation is a key feature in cancer development, and targeting CDKs has opened new avenues for cancer treatment. However, more research is required to fully exploit the therapeutic potential of CDK inhibitors and to address the challenges of drug resistance and variable efficacy across cancer types.
How CDKs Contribute to Cancer Development
Cyclin-dependent kinases (CDKs) play a pivotal role in controlling the cell cycle, ensuring the orderly progression of cells through various phases—G1, S, G2, and M phases. Their regulation is mediated by cyclins, which activate CDKs at specific points in the cycle. This tightly controlled process ensures that cell division occurs only when it is necessary and in a regulated manner. However, in cancer, the regulation of CDKs becomes dysregulated, leading to uncontrolled cell proliferation, a hallmark of tumorigenesis.
The dysregulation of CDKs in cancer can occur through various mechanisms. Genetic mutations that result in the overexpression or hyperactivation of CDKs are common. For example, mutations in CDK4 and CDK6 are frequently observed in cancers such as breast cancer and melanoma, driving excessive cell cycle progression. These mutations bypass normal regulatory checkpoints, allowing cancer cells to proliferate uncontrollably. Additionally, the amplification of cyclins such as Cyclin D1, which binds and activates CDK4/6, is a common oncogenic event that contributes to the unchecked growth of cancer cells.
Another key mechanism by which CDKs contribute to cancer development is through the inactivation of tumor suppressor pathways. The retinoblastoma protein (Rb) is a critical tumor suppressor that controls the G1/S checkpoint by inhibiting the activity of E2F transcription factors, which are essential for DNA replication. In normal cells, CDKs phosphorylate and inactivate Rb, allowing progression through the cell cycle. However, in many cancers, this regulatory pathway is disrupted. Overactive CDKs hyper-phosphorylate Rb, rendering it inactive and leading to unregulated cell cycle progression, thus promoting tumor growth.
CDKs also contribute to cancer through their interactions with other cellular processes beyond cell cycle regulation. CDK9, for instance, plays a role in regulating transcription elongation, which can influence the expression of genes involved in cancer progression. Furthermore, CDK-mediated dysregulation can affect the self-renewal and proliferation capacity of cancer stem cells, making tumors more aggressive and resistant to therapy.
The significance of CDKs in cancer development has made them attractive targets for therapeutic interventions. CDK inhibitors, such as those targeting CDK4/6, have demonstrated efficacy in slowing tumor growth by restoring control over the cell cycle. However, the complexity of CDK function in cancer suggests that targeting these kinases will require a comprehensive understanding of their role in different cancer types to develop more effective treatments.
CDKs as Biomarkers for Cancer Prognosis and Treatment
Cyclin-dependent kinases (CDKs) are gaining recognition not only for their central role in regulating the cell cycle but also as significant biomarkers for cancer prognosis and treatment. Aberrant CDK activity has been strongly associated with cancer progression, making CDKs valuable indicators for predicting disease outcomes and treatment response. The expression levels and activity of various CDKs can provide insights into tumor aggressiveness, patient prognosis, and the likelihood of responding to specific therapeutic interventions.
Several studies have shown that increased CDK expression correlates with poor clinical outcomes in various cancers. For instance, overexpression of CDK1 has been associated with shorter overall survival and increased tumor recurrence in cancers such as breast, colorectal, and bladder cancer. CDK1 plays a key role in promoting the G2/M phase transition, and its dysregulation has been linked to enhanced tumor proliferation, invasion, and metastasis. As a result, CDK1 expression is frequently used as a prognostic biomarker to assess cancer progression and aggressiveness.
In addition to CDK1, other CDKs such as CDK4 and CDK6 have been studied as prognostic markers. Elevated levels of CDK4 and CDK6 are often observed in cancers with aggressive behavior, including breast cancer and melanoma. These kinases drive cell cycle progression by phosphorylating the retinoblastoma protein (Rb), leading to the uncontrolled proliferation of cancer cells. Their overexpression is not only associated with poor prognosis but also suggests a greater likelihood of resistance to standard treatments, making them key biomarkers for identifying high-risk patients who may benefit from targeted therapies.
The clinical relevance of CDKs as biomarkers extends to their use in guiding treatment decisions. CDK inhibitors, such as palbociclib, ribociclib, and abemaciclib, which target CDK4/6, have been developed as therapeutic agents for cancers with CDK dysregulation. These inhibitors have shown significant efficacy in treating hormone receptor-positive breast cancer by inducing cell cycle arrest and reducing tumor growth. The effectiveness of CDK inhibitors is often linked to the expression levels of CDK4/6, making these kinases important predictive biomarkers for selecting patients who are likely to respond to these treatments.
Moreover, emerging research suggests that CDK inhibitors can be combined with other therapies to enhance treatment outcomes. For instance, CDK inhibition has been shown to sensitize cancer cells to DNA-damaging agents, making it a promising strategy for combination therapy in cancers with CDK dysregulation. Overall, CDKs serve as both prognostic markers and therapeutic targets, with the potential to personalize cancer treatment and improve patient outcomes.
Targeting CDKs in Cancer Therapy
Cyclin-dependent kinases (CDKs) have emerged as critical targets in cancer therapy due to their central role in regulating cell cycle progression and their frequent dysregulation in various cancers. Targeting CDKs has become a promising therapeutic strategy, particularly with the development of selective CDK inhibitors designed to halt the proliferation of cancer cells.
CDK inhibitors have been developed to specifically target key CDKs involved in cancer progression, most notably CDK4 and CDK6. These kinases drive the transition from the G1 to the S phase of the cell cycle by phosphorylating and inactivating the retinoblastoma (Rb) protein, a tumor suppressor. This phosphorylation allows cancer cells to bypass normal regulatory checkpoints and continue proliferating. By inhibiting CDK4/6, drugs like palbociclib, ribociclib, and abemaciclib effectively induce cell cycle arrest, slowing down or halting tumor growth. These inhibitors have shown significant success in treating hormone receptor-positive breast cancer, where CDK4/6 is frequently overexpressed. Palbociclib, for example, has been approved for use in combination with endocrine therapy, demonstrating an increase in progression-free survival for patients with advanced breast cancer.
Despite the success of CDK4/6 inhibitors, cancer cells can develop resistance to these therapies. Mechanisms of resistance include the loss of Rb function, mutations in CDK genes, or the activation of compensatory signaling pathways that bypass CDK4/6 inhibition. This has led researchers to explore combination therapies, where CDK inhibitors are used alongside other cancer treatments to enhance their effectiveness. For instance, combining CDK inhibitors with DNA-damaging agents or immunotherapies has shown promise in preclinical studies, as CDK inhibition can sensitize cancer cells to these treatments.
Targeting other CDKs, such as CDK7 and CDK9, which regulate transcription and RNA processing, is also being explored in clinical trials. Inhibitors targeting these kinases could be particularly effective in cancers driven by transcriptional dysregulation, such as leukemia or certain solid tumors.
In conclusion, CDK inhibitors have opened new avenues in cancer therapy, offering targeted approaches to halt tumor progression. However, overcoming resistance and improving the efficacy of combination treatments will be key challenges in maximizing their therapeutic potential.
Conclusion and Future Implications
Cyclin-dependent kinases (CDKs) are central to the regulation of the cell cycle, and their dysregulation is a hallmark of many cancers. Over the past decade, the role of CDKs in tumorigenesis has been increasingly recognized, not only for their involvement in cell cycle control but also in transcriptional regulation and epigenetic mechanisms. CDKs have therefore emerged as valuable biomarkers for cancer prognosis and as promising targets for therapeutic intervention.
The success of CDK inhibitors, particularly those targeting CDK4/6, such as palbociclib and ribociclib, has significantly impacted the treatment landscape for hormone receptor-positive breast cancer. These inhibitors have extended progression-free survival in patients, showcasing the potential of CDK-targeted therapies in clinical oncology. Despite these advances, challenges remain, particularly in addressing the mechanisms of resistance that cancer cells develop to CDK inhibitors. Understanding these resistance pathways and identifying biomarkers that predict responsiveness to CDK inhibitors will be crucial for optimizing treatment strategies.
Since activity of CDKs is associated with induction of stem cell properties, drugs targeting these proteins might be used for efective elimination of cancer stem cells and reduction of tumor metastases. Tis implicates that CDKs are involved in the pathogenesis of a high spectrum of cancers, including diferent types of carcinomas as well as non-epithelial malignancies. Coming from this point of view CDKs will come more and more in the focus as therapeutical targets. Activity levels of CDKs can be used for prediction of cancer prognosis and response of patients to various therapeutic options. In fact, an appropriate approach for implementation of personalized medicine in the feld of cancer therapy is measurement of activity of these proteins.
Cumulatively, CDKs represent ideal therapeutic targets for cancer. Tus, future studies should focus on assessment of their activities in diferent tumors and identifcation of their association with clinicopathological data.
Moreover, the presence of putative genetic variants within CDK coding genes might afect their activity and susceptibility of persons to diferent cancers. Tis note should also be assessed in future studies.
References
- Ghafouri-Fard, S., Khoshbakht, T., Hussen, B. M., Dong, P., Gassler, N., Taheri, M., Baniahmad, A., & Dilmaghani, N. A. (2022). A review on the role of cyclin dependent kinases in cancers. Cancer Cell International, 22(325).
- Otto, T., & Sicinski, P. (2017). Cell cycle proteins as promising targets in cancer therapy. Nature Reviews Cancer, 17(2), 93-115.
- Malumbres, M., & Barbacid, M. (2009). Cell cycle, CDKs and cancer: A changing paradigm. Nature Reviews Cancer, 9(3), 153-166.
- Hydbring, P., Malumbres, M., & Sicinski, P. (2016). Non-canonical functions of cell cycle cyclins and CDKs. Nature Reviews Molecular Cell Biology, 17(5), 280-292.
- Dickson, M. A. (2014). Molecular pathways: CDK4 inhibitors for cancer therapy. Clinical Cancer Research, 20(13), 3379-3383.
- Asghar, U., Witkiewicz, A. K., Turner, N. C., & Knudsen, E. S. (2015). The history and future of targeting cyclin-dependent kinases in cancer therapy. Nature Reviews Drug Discovery, 14(2), 130-146.
- VanArsdale, T., Boshoff, C., Arndt, K. T., & Abraham, R. T. (2015). Molecular pathways: Targeting the cyclin D–CDK4/6 axis for cancer treatment. Clinical Cancer Research, 21(13), 2905-2910.
- Johnson, N., & Shapiro, G. I. (2018). Cyclin-dependent kinases (CDKs) and the DNA damage response: Rationale for CDK inhibitor–chemotherapy combinations as an anticancer strategy. Journal of Clinical Oncology, 36(3), 285-289.
- Turner, N. C., Ro, J., André, F., Loi, S., Verma, S., Iwata, H., & Harbeck, N. (2015). Palbociclib in hormone-receptor–positive advanced breast cancer. The New England Journal of Medicine, 373(3), 209-219.