The status and challenges of thalidomide and its analogues
Abstract
Thalidomide is a glutamic acid derivative that was used as a sedative in over 40 countries worldwide in the 1950s due to its sedative, antiemetic, and hypnotic effects. It was widely used to alleviate early pregnancy symptoms in pregnant women but was withdrawn from the market after causing thousands of cases of phocomelia. Despite its withdrawal from the market, research on thalidomide continued. Occasionally, it was discovered that the drug has a therapeutic effect on a skin inflammation leprosy erythema nodosum caused by high levels of TNF in serum. In addition to its anti-inflammatory effects, researchers also found that thalidomide has anti-angiogenic and immune-modulatory effects, including T cell co-stimulation and activation of NK cells. This finding made thalidomide become a hot topic in cancer treatment research.
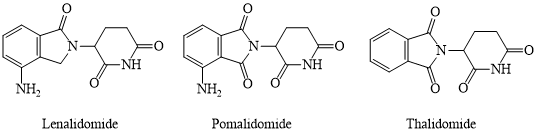
The US company Celgene has initiated a research program to develop derivatives of thalidomide in search of drugs with stronger efficacy and fewer side effects to replace thalidomide. So far, structural analogues of thalidomide, lenalidomide, and pomalidomide, have been approved by the FDA for the treatment of multiple myeloma and have improved the survival status of countless myeloma patients. However, we still know little about their mechanisms of action. Due to their serious side effects, such drugs remain under strict regulation. Therefore, identifying the detailed molecular mechanism of thalidomide and its analogues is particularly important.
What is the target of thalidomide?
As an immunomodulatory drug, the direct target of thalidomide has been unknown until 2010, Ito et al.found that its main target was CRBN. The mutation of CRBN gene is associated with autosomal recessive non-syndromic mental retardation, which may be caused by the disorder of calcium-activated potassium channel in the brain. CRBN forms an E3 ubiquitin ligase complex with DNA damage binding protein 1 (DDB1), cullin-4A (CUL4A), and the regulatory protein ROC1 of cullins 1. CRBN binds to the substrate protein in this complex, thereby ubiquitinating the substrate and promoting the degradation of the target protein.
What is the action mechanism of thalidomide and its analogues?
As the earliest direct target of thalidomide, CRBN has become the focus of research on thalidomide and immunomodulatory drugs. Studies have shown that the anti-multiple myeloma (MM) activity of the immunomodulatory drug thalidomide is achieved through CRBN, and the effect of drug treatment MM is positively correlated with CRBN. Thalidomide promoted the ubiquitination and proteasome degradation of IKZF1 and IKZF3 by binding to CRBN. IKZF1 and IKZF3 are important transcription factors for B cell differentiation. An amino acid substitution of IKZF3 can resist the degradation induced by thalidomide and the inhibition of thalidomide on the growth of MM cells, further indicating that the inhibition of these transcription factors may be the mechanism of thalidomide in the treatment of MM.
What is CRBN?
CRBN (Cereblon) is a protein encoded by the CRBN gene located on human chromosome 3, which is expressed in many different tissues and cell types. CRBN protein is composed of 442 amino acids with a molecular weight of about 51 k Da. CRBN protein is part of the complex that plays a key role in the ubiquitin-proteasome system and is responsible for regulating the levels of many proteins in cells. Specifically, CRBN is an E3 ubiquitin ligase that works with E2 ubiquitin-conjugating enzymes to label target proteins and further degrade them through the proteasome pathway.
CRBN has become an important target in cancer therapy due to its role in the degradation of certain proteins that are critical for cancer cell survival. In particular, the CRBN complex is involved in the degradation of the transcription factor Ikaros and the oncoprotein Aiolos, which are both important for the survival and proliferation of multiple myeloma cells.
The idea of protein degradation targeting chimeras was proposed by Craig Crews and his colleagues at Yale University in the late 1990s. They hope to develop a new protein degradation method to overcome the limitations of traditional small molecule inhibitors. They envisioned a molecule that recruits an E3 ubiquitin ligase to a specific protein of interest and promotes its degradation by the proteasome. The first proof-of-concept studies for PROTACs were published in 2001, and since then, the technology has been refined and developed by many different research groups. The in-depth study found that CRBN can bind to related proteins to form an E3 ubiquitin ligase complex, further bind to substrate proteins, ubiquitinate substrates and promote the degradation of target proteins. Because the direct target of thalidomide, lenalidomide and pomalidomide is the CRBN receptor, it can be recruited to the corresponding target protein by directly binding to the CRBN protein to play the role of ubiquitination and proteasome degradation, extensive attention.
What are the advantages of thalidomide, lenalidomide and pomalidomide as CRBN ligands applied to PROTACs?
Thalidomide, lenalidomide, and pomalidomide directly target the CRBN receptor. They have a high affinity for the CRBN protein, which can be recruited to the corresponding target protein by directly binding to the CRBN protein to play the role of ubiquitination and proteasome degradation. CRBN protein is an essential component of the ubiquitin-proteasome system for protein degradation of PROTAC.
PROTAC molecules are composed of three parts: small molecule ligands that recruit specific target proteins, E3 ligase ligands, and linker chains that connect the two. Currently, there are thousands of ligands with potential as E3 ligase ligands, but only a few can be used in practice. Among them, CRBN ligands thalidomide, pomalidomide, and lenalidomide have become one of the main E3 ligase ligands in the design of PROTAC molecules due to their high affinity for proteins and simple synthesis steps, as well as low economic costs.
Summary
After thalidomide and similar drugs were approved to treat various bone marrow cancers, their efficacy in cancer treatment caught the attention of researchers. As a result, many researchers began investigating thalidomide’s mechanism of action and discovered that it directly targets the CRBN protein. Further exploration revealed that CRBN can form an E3 ubiquitin ligase complex with related proteins in the body, which binds to substrate proteins and promotes the degradation of the target protein through ubiquitination. This breakthrough has paved the way for the development and design of PROTAC technology. CRBN ligands have since become a crucial component of PROTAC molecular design.
Reference
- Fischer, E. S., Böhm, K., Lydeard, J. R., Yang, H., Stadler, M. B., Cavadini, S., … & Thomä, N. H. (2014). Structure of the DDB1–CRBN E3 ubiquitin ligase in complex with thalidomide. Nature, 512(7512), 49-53.
- Wang, C., Zhang, Y., Wu, Y., & Xing, D. (2021). Developments of CRBN-based PROTACs as potential therapeutic agents. European Journal of Medicinal Chemistry, 225, 113749.
- Barankiewicz, J., Salomon-Perzyński, A., Misiewicz-Krzemińska, I., & Lech-Marańda, E. (2022). CRL4CRBN E3 Ligase Complex as a Therapeutic Target in Multiple Myeloma. Cancers, 14(18), 4492.
- Yamamoto, J., Ito, T., Yamaguchi, Y., & Handa, H. (2022). Discovery of CRBN as a target of thalidomide: A breakthrough for progress in the development of protein degraders. Chemical Society Reviews.