Therapeutic Potential of Targeting Cgas-Sting Pathway in Disease
Abstract
The cGAS-STING signaling pathway is a crucial innate immune pathway that recognizes and responds to cytosolic DNA, indicating cellular damage or infection. cGAS senses cytosolic DNA, producing cyclic GMP-AMP (cGAMP) that activates STING. This leads to downstream signaling, causing the production of type I interferons and cytokines, promoting an antiviral and inflammatory response. Dysregulation of the cGAS-STING pathway is linked to human diseases such as cancer, autoimmune disorders, and viral infections, making it an attractive target for therapeutic interventions.
Introduction of cGAS-STING signaling pathway
The activation of the cGAS-STING signaling pathway is a crucial component of the innate immune system, playing a vital role in the host’s defense against viral and bacterial infections. This pathway is triggered by cytoplasmic DNA, which is detected by cyclic GMP-AMP synthase (cGAS), catalyzing the synthesis of cyclic GMP-AMP (cGAMP) from ATP and GTP. cGAMP subsequently binds to the stimulator of interferon genes (STING), leading to the activation of downstream signaling pathways and the production of type I interferons and inflammatory cytokines.
However, recent studies have highlighted the significant role of the cGAS-STING pathway in regulating antitumor immunity. These findings have illuminated the molecular mechanisms and structures of the pathway, providing valuable insights into its potential as a therapeutic target for infectious diseases and cancer. These developments have brought new hope for the development of novel cancer immunotherapies targeting the cGAS-STING pathway.
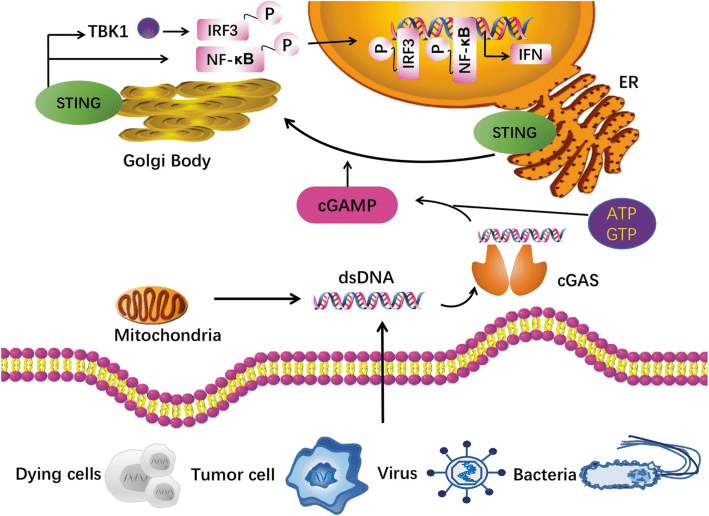
Fig. 1[1] cGAS-STING pathway.
Structural Basis of cGAS Activation
The structure of cGAS has been extensively studied and it has been shown to adopt a dimeric conformation upon binding to DNA. This conformational change is necessary for cGAS activation and cGAMP production. Recent studies have also revealed the mechanism of cGAS activation by DNA, which involves DNA binding-induced conformational changes that are propagated to the active site of the enzyme. The crystal structure of cGAS in complex with DNA has also revealed the key residues involved in DNA recognition and catalysis.
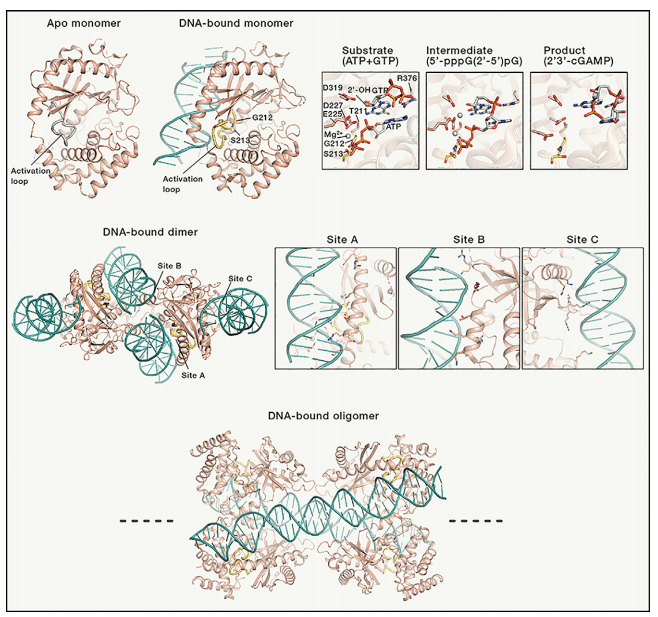
Figure 2[3]. Structures of cGAS in various states
STING Signaling and Downstream Responses
STING is a transmembrane protein that is located on the endoplasmic reticulum (ER) membrane. Upon activation, STING undergoes a conformational change that exposes its C-terminal tail to the cytoplasm, where it recruits and activates downstream signaling molecules. This leads to the activation of transcription factors such as IRF3 and NF-κB, which induce the production of type I interferons and other pro-inflammatory cytokines. The downstream responses to STING activation are critical for the immune response to viral infections and cancer cells.
Mechanisms of STING Activation and Signaling
The mechanisms of STING activation and signaling have been extensively studied and several key proteins have been identified that play important roles in this pathway. These include TBK1, which phosphorylates and activates IRF3, and TRAF6, which activates NF-κB signaling. Recent studies have also revealed the structural basis of STING activation by cGAMP, which involves the binding of cGAMP to a specific binding pocket on the STING protein. This induces a conformational change in STING that activates downstream signaling.
Conformational Changes during cGAS-STING Activation
Recent research has shed light on the conformational changes that take place during cGAS-STING activation, providing important understanding of the molecular mechanisms involved. These studies indicate that cGAS binding to DNA triggers a conformational shift in cGAS, which is then passed on to STING, resulting in its activation. This change is crucial for the downstream signaling and immune response against viral infections and cancer cells.
Role of cGAS-STING in cancer immunity
Therapeutic targeting of the cGAS-STING pathway has emerged as a promising approach for cancer immunotherapy. Preclinical studies have shown that activation of the cGAS-STING pathway enhances the efficacy of immunotherapy and promotes anti-tumor immune responses. These findings have led to increased interest in the cGAS-STING pathway as a potential target for cancer therapy and highlight the importance of further research in this area.
The cGAS-STING signaling pathway has emerged as a crucial player in cancer immunity. The activation of this pathway in response to cytoplasmic DNA from tumors triggers the production of type I interferons and other pro-inflammatory cytokines, leading to the recruitment and activation of immune cells that can target and destroy cancer cells.
The cGAS-STING pathway is involved in the activation of both innate and adaptive immune responses against tumors. In the innate immune system, cGAS recognizes cytosolic dsDNA derived from tumor cells, leading to the production of cyclic GMP-AMP (cGAMP) and subsequent activation of the STING pathway. This, in turn, leads to the production of type I interferons (IFNs) and other proinflammatory cytokines, resulting in the recruitment and activation of immune cells such as natural killer cells, dendritic cells, and T cells.
In the adaptive immune system, the cGAS-STING pathway is involved in the priming of CD8+ T cells, which play a critical role in antitumor immunity. STING signaling in dendritic cells is necessary for the activation of CD8+ T cells, and STING agonists have been shown to enhance the antitumor activity of CD8+ T cells.
Recent studies have shown that activation of the cGAS-STING pathway in cancer cells can promote tumor regression by enhancing the immune response against the tumor. Furthermore, activation of the cGAS-STING pathway can sensitize tumor cells to immunotherapy, such as immune checkpoint inhibitors or adoptive T-cell therapy, by increasing tumor antigen presentation and reducing immune suppression in the tumor microenvironment.
Overall, the cGAS-STING pathway has the potential to be a promising target for cancer immunotherapy, as it plays a critical role in initiating and regulating immune responses against tumors.
cGAS-STING signaling in infection
The cGAS-STING pathway is a critical component of the innate immune system that plays a crucial role in detecting and responding to viral and bacterial infections. The pathway is activated when cGAS recognizes the presence of cytosolic DNA from invading pathogens, such as viruses or bacteria. Upon viral infection, cGAS specifically detects viral DNA, and produces the second messenger cyclic GMP-AMP (cGAMP). cGAMP then binds to and activates the STING protein, triggering downstream signaling events that culminate in the expression of type I interferons and other cytokines. This promotes an antiviral response, leading to the clearance of the virus.
In addition to viral DNA, bacterial DNA can also activate the cGAS-STING pathway, leading to inflammation and the recruitment of immune cells. However, some bacteria have evolved mechanisms to evade cGAS-STING detection, enabling them to persist and cause disease. For example, some bacteria encode specific enzymes that degrade cGAMP, while others secrete proteins that bind to or modify STING, preventing its activation. These bacterial evasion mechanisms highlight the importance of the cGAS-STING pathway in the host defense against infection.
Understanding the mechanisms of cGAS-STING activation and regulation during infection is crucial for developing effective therapies to treat infectious diseases. Targeting this pathway has the potential to enhance the host response to infections or suppress excessive inflammation associated with some viral infections.
cGAS-STING signaling in cancer immunotherapy
The activation of the cGAS-STING pathway has been shown to enhance the efficacy of several cancer immunotherapy approaches, including checkpoint blockade therapy, adoptive T-cell therapy, and cancer vaccines. STING agonists have been used in preclinical models to enhance the efficacy of these therapies by promoting the activation and recruitment of immune cells to the tumor microenvironment.
Checkpoint blockade therapy, which involves blocking inhibitory receptors on T cells, has shown remarkable success in the treatment of certain types of cancer. However, not all patients respond to this therapy, and resistance often develops. STING agonists have been shown to enhance the response to checkpoint blockade therapy by promoting the activation of T cells and increasing the production of type I IFNs, which can sensitize tumors to the effects of checkpoint inhibitors.
Adoptive T-cell therapy involves the isolation and expansion of tumor-specific T cells, which are then infused back into the patient to target and kill tumor cells. STING agonists have been shown to enhance the efficacy of adoptive T-cell therapy by promoting the recruitment and activation of T cells in the tumor microenvironment.
Cancer vaccines are designed to elicit an immune response against tumor antigens. STING agonists have been used in preclinical models to enhance the efficacy of cancer vaccines by promoting the activation of dendritic cells and the production of type I IFNs.
Moreover, cGAS-STING activation can also sensitize tumor cells to conventional therapies such as chemotherapy and radiation therapy, by increasing the immunogenicity of tumor cells and promoting the release of damage-associated molecular patterns (DAMPs) that can further enhance the immune response against the tumor.
However, the clinical translation of cGAS-STING activation as a therapeutic strategy for cancer treatment faces several challenges, such as developing safe and effective delivery systems for cGAS-STING agonists and identifying patient populations that are most likely to benefit from this approach. Nevertheless, the promising results from preclinical studies suggest that cGAS-STING activation could be a valuable addition to the armamentarium of cancer immunotherapies.The cGAS-STING signaling pathway provides a new opportunity for overcoming the obstacles in the application of immunotherapy (Table 1).
Table 1[2] The antitumor effect of cGAS-STING agonists.
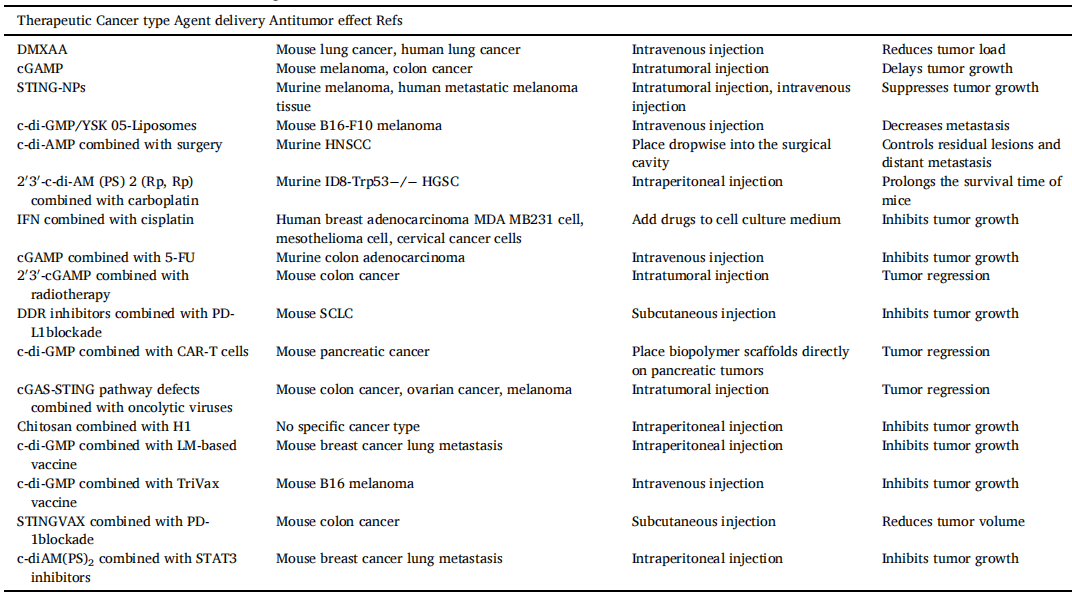
Abbreviation: DMXAA, 5, 6-dimethylxanthenone-4-acetic acid; cGAMP, cyclic GMP-AMP; STING-NPs, STING-activating nanoparticles; HNSCC, head and neck squamous cell carcinoma; LM-based vaccine, Listeria monocytogenes-based vaccine; MDSCs, Myeloid-derived suppressor cells.
Targeting cGAS-STING for cancer treatment
Targeting the cGAS-STING signaling pathway is a promising strategy for cancer treatment due to its ability to promote anti-tumor immune responses and sensitize tumor cells to conventional therapies. One approach is to use small-molecule agonists that activate cGAS and STING, which have shown anti-tumor activity in preclinical studies. However, optimizing drug delivery and minimizing off-target effects remain significant challenges.
Another approach is to use immunotherapies that stimulate the immune system to activate the pathway. Immune checkpoint inhibitors targeting PD-1 or CTLA-4 have been shown to activate the cGAS-STING pathway in tumors and promote anti-tumor immune responses. Combination therapies targeting both the cGAS-STING pathway and other immune checkpoints or conventional therapies have also shown promising results in preclinical studies and clinical trials.
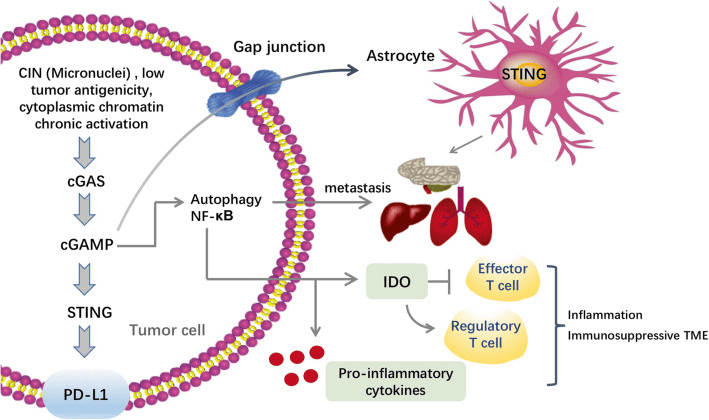
Fig. 3[1] Regulation of cGAS-STING pathway in tumor promotion
Additionally, inhibitors of negative regulators of the pathway and genetic approaches have been explored for targeting the cGAS-STING pathway in cancer treatment. However, the development of effective STING agonists for clinical use has been challenging, as some agonists can induce toxicity or off-target effects.
Limitations and side effects of targeting cGAS-STING
Despite the promising potential of targeting cGAS-STING in cancer immunotherapy, there are also limitations and potential side effects. One major limitation is that cGAS-STING activation may trigger an excessive immune response, leading to autoimmune diseases or inflammation.
Another challenge is to identify the most effective and specific agents for cGAS-STING activation. For example, the choice of CDN agonists and delivery methods can significantly impact the efficacy of cGAS activation and the resulting anti-tumor immune response.
Future directions for cGAS-STING research in cancer immunotherapy
To enhance the effectiveness of cGAS-STING targeting in cancer immunotherapy, it is crucial to conduct further research to comprehend the molecular mechanisms and regulatory factors underlying cGAS-STING signaling.
Additionally, developing new agents with better specificity and efficacy for cGAS-STING activation is imperative. Moreover, it is crucial to explore the optimal combination of cGAS-STING targeting with other immunotherapeutic approaches like checkpoint inhibitors or CAR-T cell therapy to improve the anti-tumor immune response and the clinical outcomes for cancer patients.
References