Tirzepatide : a New Era of Dual-Targeted Treatment for Diabetes and Obesity
Abstract
Tirzepatide marks a significant advancement in the treatment of two intertwined global health issues: diabetes and obesity. As the prevalence of these conditions continues to rise, particularly in developed countries, innovative treatments like Tirzepatide provide a new hope. This drug is notable for its dual mechanism of action, targeting both the glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptors, which makes it a potent therapeutic option for reducing blood glucose levels and body weight.
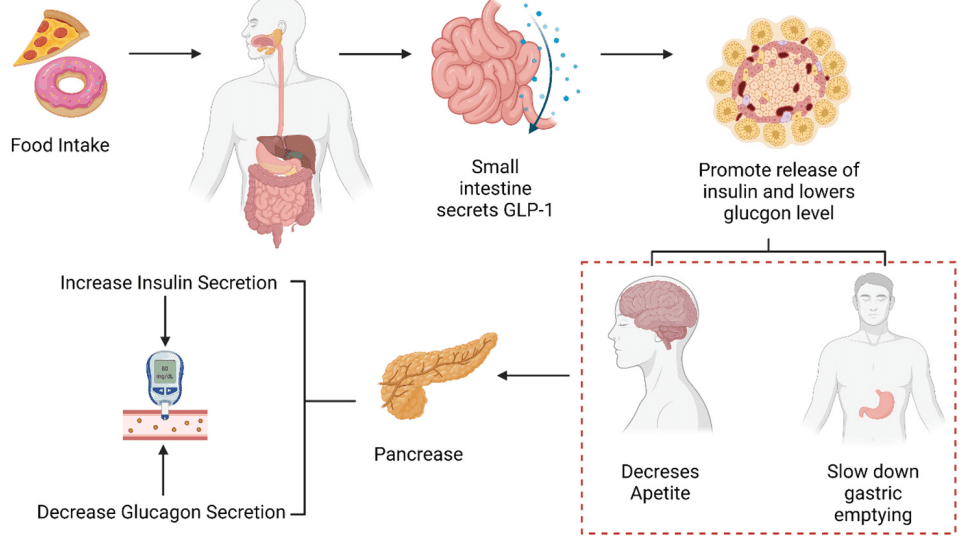
Figure 1. Role of GLP-1 in glucose metabolism.
The Twin Epidemics: Diabetes and Obesity
Diabetes and obesity are often referred to as twin epidemics due to their frequent co-occurrence and the combined health burden they impose. Type 2 diabetes (T2D), which typically develops as a result of obesity and poor lifestyle choices, leads to significant morbidity and mortality. Managing these conditions requires innovative pharmacological interventions alongside lifestyle modifications.
Obesity is a major risk factor for numerous health problems, including diabetes, hypertension, and cardiovascular disease. The World Health Organization (WHO) defines obesity based on the body mass index (BMI), with a BMI over 30 kg/m^2 categorizing an adult as obese. Efforts to combat obesity have traditionally focused on diet and exercise, but these measures are often insufficient for long-term management.
Rise of Type 2 Diabetes
Type 2 diabetes is characterized by insulin resistance and beta-cell dysfunction, which leads to high blood sugar levels. Over the past few decades, the prevalence of T2D has increased dramatically, making it a critical public health challenge. Traditional treatments have included lifestyle interventions and medications like metformin, but these often fail to provide sustained glycemic control and come with various side effects.
Emerging Treatments
In response to the growing need for effective treatments, Tirzepatide has been developed as a synthetic peptide that can activate both GLP-1 and GIP receptors. This dual activity is believed to enhance the drug’s effectiveness in controlling blood sugar and promoting weight loss compared to treatments that target a single receptor.
Pharmacodynamics of Tirzepatide and the Incretin Effect
Dual Mechanism of Tirzepatide
Tirzepatide stands out in the treatment of diabetes and obesity due to its innovative dual-action mechanism. It functions as both a glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptor agonist. This combination allows Tirzepatide to effectively lower blood glucose levels while also aiding in weight loss—a critical need in the management of type 2 diabetes and obesity.
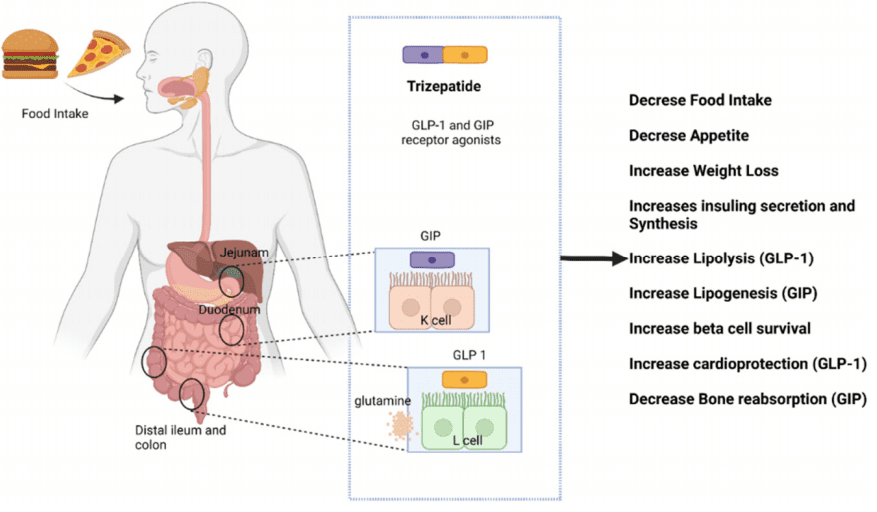
Figure 2. Mechanisms of action of tirzepatide within the human body
The Role of Incretins in Glucose Metabolism
Incretins, primarily GLP-1 and GIP, are hormones that play a vital role in regulating glucose metabolism. They are released postprandially from the gut and stimulate insulin secretion in a glucose-dependent manner. The ‘incretin effect’ refers to the enhanced insulin secretion seen after oral glucose intake compared to intravenous glucose delivery, highlighting the significance of the gastrointestinal tract in glucose regulation.
GLP-1 and GIP Functions
GLP-1: Enhances insulin secretion, inhibits glucagon release, slows gastric emptying, and decreases appetite and food intake. GLP-1 receptor agonists have been effectively used in diabetes management, primarily affecting glucose control and weight loss.
GIP: Also stimulates insulin secretion but has been less utilized therapeutically due to less pronounced effects in patients with type 2 diabetes. However, when combined with GLP-1 activity, as in Tirzepatide, the synergistic effects can enhance overall metabolic control.
Benefits of Tirzepatide Over Traditional Treatments
The dual action of Tirzepatide provides a more comprehensive approach than previous medications that target only one of these pathways. This dual targeting leads to more significant improvements in glucose control and body weight reduction, making Tirzepatide a promising option for patients who have struggled with other treatments. Its ability to act on two fronts helps to address the multifaceted challenges presented by diabetes and obesity more effectively.
Incretin Mimetics and Their Evolution
Tirzepatide is part of a newer class of diabetic medications known as incretin mimetics. These drugs mimic the action of incretins and have revolutionized the treatment of type 2 diabetes by offering not only improved glycemic control but also beneficial effects on weight loss and cardiovascular risk factors.
The development of incretin mimetics began with the discovery of the incretin effect in the early 1970s. The realization that gut-derived hormones could enhance insulin secretion led to the exploration of therapeutic agents that could exploit this pathway. The introduction of GLP-1 receptor agonists marked the first step, and the development of dual-acting agents like Tirzepatide represents the latest advancement in this field.
Clinical Development and Trials of Tirzepatide
Overview of Clinical Trials
The clinical development of Tirzepatide has been comprehensive, encompassing multiple phases that assess efficacy, safety, and tolerability across diverse patient populations. These trials have consistently demonstrated Tirzepatide’s ability to significantly improve glycemic control and promote weight loss, positioning it as a groundbreaking treatment in the management of type 2 diabetes and obesity.
Phase 1 and 2 Clinical Trials
Initial phase 1 trials of Tirzepatide focused on safety and pharmacokinetics, revealing a favorable profile that supported further investigation. Subsequent phase 2 trials expanded to evaluate its effectiveness in lowering blood glucose levels and body weight. These studies confirmed that Tirzepatide improves the management of blood glucose with an added benefit of substantial weight reduction compared to existing treatments.
Key Findings
HbA1c Reduction: Tirzepatide showed a remarkable ability to decrease HbA1c, a measure of blood glucose management, surpassing results seen with other incretin-based therapies.
Weight Loss: Participants in the trials experienced significant weight loss, a crucial advantage for many patients with type 2 diabetes who are also overweight or obese.
Phase 3 Clinical Trials: SURPASS Program
The SURPASS program, encompassing several global phase 3 trials, further evaluated the efficacy and safety of Tirzepatide. These trials involved a broad spectrum of patients, including those newly diagnosed with diabetes, those inadequately controlled on existing medication, and those managing both diabetes and obesity.
SURPASS Highlights
Comprehensive Efficacy: Tirzepatide significantly improved glycemic control and facilitated weight loss across all patient groups. Its effects were robust, often outperforming leading GLP-1 receptor agonists in head-to-head comparisons.
Safety Profile: The treatment was well-tolerated, with gastrointestinal symptoms being the most commonly reported side effects. These were generally mild to moderate in severity and tended to decrease over time.
Additive Benefits: In combination with other treatments like SGLT2 inhibitors, Tirzepatide provided additional glycemic and weight management benefits.
Comparative Effectiveness
Tirzepatide’s dual mechanism provides a distinct advantage over other diabetic medications that only target one incretin pathway. The SURPASS-2 trial, for instance, showed that Tirzepatide was superior to semaglutide in reducing HbA1c levels and body weight. These findings underscore Tirzepatide’s potential as a transformative therapy for patients with type 2 diabetes, especially those struggling with obesity.
Long-term Implications
The clinical success of Tirzepatide not only enhances diabetes treatment but also helps mitigate the associated risks of cardiovascular diseases and other complications related to obesity. Its ability to improve multiple health outcomes simultaneously represents a significant leap forward in managing chronic conditions that often coexist.
Broader Implications and Future Directions for Tirzepatide
Impact on Public Health
Tirzepatide’s approval and clinical application represent a significant advancement in public health, particularly in the realms of diabetes and obesity management. With the increasing prevalence of these conditions globally, Tirzepatide offers a promising solution that addresses both issues simultaneously, potentially reducing the burden on health systems caused by the complications associated with poorly managed diabetes and obesity.
Pharmacokinetics and Patient Convenience
One of the standout features of Tirzepatide is its pharmacokinetic profile, which supports a once-weekly dosing schedule. This is particularly beneficial for patient compliance and quality of life, as managing diabetes often involves complex treatment regimens that can be burdensome. Tirzepatide’s extended half-life and steady-state exposure allow for consistent therapeutic levels with less frequent dosing compared to daily diabetes medications.
Key Pharmacokinetic Attributes
Dose Proportionality: Tirzepatide shows a dose-proportional increase in plasma concentration, making it easier to adjust doses based on patient response and tolerance.
Extended Half-Life: The drug’s half-life of approximately 5 days supports weekly administration, which helps maintain consistent drug levels in the body, enhancing both efficacy and patient adherence.
Future Research and Development
While Tirzepatide has already shown substantial benefits, ongoing and future research will likely focus on further elucidating its long-term effects, particularly in cardiovascular health, renal function, and potential protective effects against other metabolic syndromes. Moreover, exploring its use in populations with specific comorbidities, such as severe obesity, cardiovascular disease, or those with different ethnic backgrounds that may exhibit varying responses to treatment, will be crucial.
Areas of Focus for Ongoing Studies
Long-term Cardiovascular Outcomes: Given the link between diabetes, obesity, and cardiovascular disease, understanding Tirzepatide’s impact on heart health over extended periods is vital.
Combination Therapy Potential: Investigating Tirzepatide’s effectiveness when used in combination with other therapeutic agents could open new avenues for comprehensive metabolic syndrome management.
Mechanistic Studies: Further studies to understand the molecular mechanisms behind Tirzepatide’s action could lead to the development of even more targeted therapies.
Integration into Clinical Practice
As Tirzepatide becomes more widely used in clinical settings, healthcare providers will need to consider the best strategies for integrating this new treatment into existing therapeutic regimens. This will involve training medical professionals on the unique aspects of Tirzepatide, including its dual mechanism of action, benefits, side effects, and the specific patient populations who might benefit the most from its use.
Tirzepatide is a groundbreaking therapeutic agent that significantly advances the treatment of type 2 diabetes and obesity, offering improved glycemic control, weight reduction, and potential cardiometabolic benefits. Its introduction is timely, given the rising global incidence of these chronic conditions. As we move forward, Tirzepatide will not only enhance the quality of life for many patients but also reduce the long-term public health burden associated with diabetes and obesity. Continued research and clinical practice integration will be essential to fully realize its potential and optimize outcomes for patients worldwide.
Reference
- Chavda, V. P., Ajabiya, J., Teli, D., Bojarska, J., & Apostolopoulos, V. (2022). Tirzepatide, a new era of dual-targeted treatment for diabetes and obesity: a mini-review. Molecules, 27(13), 4315.
- Abdelaal, M., le Roux, C. W., & Docherty, N. G. (2017). Morbidity and mortality associated with obesity. Annals of translational medicine, 5(7).
- Alam, S., Hasan, M. K., Neaz, S., Hussain, N., Hossain, M. F., & Rahman, T. (2021). Diabetes Mellitus: insights from epidemiology, biochemistry, risk factors, diagnosis, complications and comprehensive management. Diabetology, 2(2), 36-50.
- Zeynaloo, E., Stone, L. D., Dikici, E., Ricordi, C., Deo, S. K., Bachas, L. G., … & Lanzoni, G. (2022). Delivery of therapeutic agents and cells to pancreatic islets: Towards a new era in the treatment of diabetes. Molecular aspects of medicine, 83, 101063.
- Wang, L. (2023). Designing a dual GLP-1R/GIPR agonist from tirzepatide: comparing residues between tirzepatide, GLP-1, and GIP. Drug design, development and therapy, 1547-1559.