Tovorafenib: A Breakthrough in Treating Pediatric Low-Grade Gliomas
Abstract
Tovorafenib is a promising new treatment for pediatric low-grade gliomas (pLGG), a type of slow-growing brain tumor that commonly affects children. As a selective RAF kinase inhibitor, tovorafenib targets specific genetic mutations, particularly those in the BRAF gene, which are often found in pLGGs. This drug represents a more targeted, effective, and less toxic alternative to traditional chemotherapy. Early clinical trials, including FIREFLY-1 and FIREFLY-2, have shown encouraging results, with many patients experiencing tumor shrinkage or even complete remission. While the drug’s side effects are generally manageable, serious risks such as liver toxicity and growth delays have been observed in some cases. Tovorafenib has received Breakthrough Therapy designation from the FDA, signaling its potential to transform pediatric cancer treatment. As research continues, the future of tovorafenib looks promising, offering hope for children with BRAF-driven brain tumors and potentially paving the way for targeted therapies in other cancers.
Every year, thousands of children around the world are diagnosed with brain tumors. For some, treatment options are limited, and the road to recovery is a difficult one. However, a promising new drug called tovorafenib offers hope, particularly for children diagnosed with pediatric low-grade gliomas (pLGG). This drug is revolutionizing the treatment of brain tumors by targeting specific mutations, offering a more effective and less toxic alternative to traditional therapies.
Tovorafenib is a RAF kinase inhibitor that works by blocking a key enzyme in the MAPK signaling pathway, which plays a crucial role in cancer cell growth. Currently undergoing clinical trials, tovorafenib has already demonstrated significant potential for treating pLGG, a slow-growing brain tumor that predominantly affects children. For families facing the uncertainty of brain cancer, tovorafenib offers a new avenue of hope.
In this post, we’ll explore what tovorafenib is, how it works, the clinical trials behind it, its side effects, and why it matters in the broader landscape of pediatric cancer treatment.
Understanding Pediatric Low-Grade Gliomas
Pediatric low-grade gliomas (pLGGs) are a type of brain tumor that originates from glial cells, which provide support and nourishment to neurons. These tumors are considered “low-grade” because they grow slowly compared to high-grade gliomas, which are more aggressive. Despite their slower progression, pLGGs can still lead to significant health issues, particularly when located in critical regions of the brain.
In children, pLGGs make up about 30% of all brain tumors, and they are often diagnosed in children under 10 years old. The exact cause of pLGGs remains unclear, but researchers have identified a strong correlation between genetic mutations, particularly in the BRAF gene, and the development of these tumors.
The BRAF gene encodes a protein that plays a role in regulating cell growth. When this gene mutates, it can lead to unchecked cell division and, ultimately, tumor formation. These mutations are common in pLGGs, making them an ideal target for treatments like tovorafenib, which specifically targets and blocks the activity of the BRAF protein.
Traditional treatments for pLGG, such as surgery and chemotherapy, can be effective, but they often come with significant side effects and risks, especially in children. This is where tovorafenib offers a new and potentially safer alternative by directly targeting the molecular cause of the tumor.
What is Tovorafenib?
Tovorafenib, marketed under the brand name Ojemda, is an oral RAF kinase inhibitor designed to target specific genetic mutations in pLGG. It works by blocking the activity of the RAF protein, which is a key component of the MAPK pathway, a signaling cascade that regulates various cellular processes, including growth and survival. In many cancers, this pathway becomes overactive, leading to uncontrolled cell proliferation and tumor growth.
By inhibiting the RAF protein, tovorafenib prevents the activation of downstream signaling in the MAPK pathway, effectively halting the growth of tumor cells that rely on this pathway for survival. What makes tovorafenib particularly powerful is its ability to specifically target BRAF mutations, which are prevalent in many pediatric low-grade gliomas.
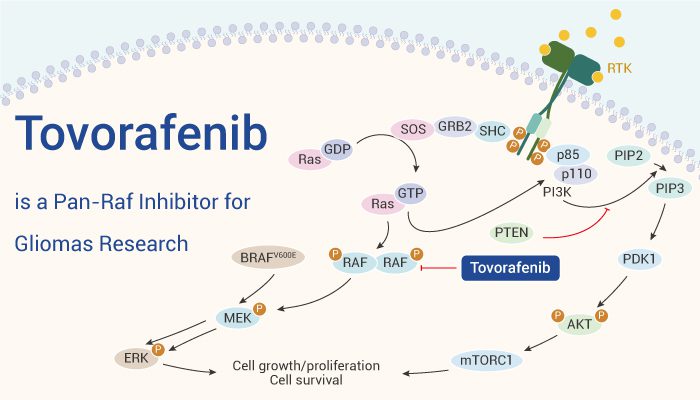
Fig.1 What is Tovorafenib.
This targeted approach is a departure from traditional cancer treatments like chemotherapy, which indiscriminately kill both cancerous and healthy cells. Chemotherapy can lead to severe side effects, such as nausea, hair loss, and weakened immune function. In contrast, tovorafenib offers a more precise treatment with potentially fewer side effects, as it is specifically designed to target the molecular alterations driving tumor growth in certain cancer cells.
Tovorafenib is also notable for its ability to penetrate the blood-brain barrier, which is crucial for treating brain tumors. This means the drug can effectively reach and act on tumors within the brain, an area that has historically been difficult to treat with oral medications.
Clinical Trials and Results
Tovorafenib has shown promising results in several clinical trials, particularly in the treatment of pediatric low-grade gliomas with BRAF mutations. The most notable of these is the FIREFLY-1 study, which evaluated the drug’s safety and efficacy in children with advanced BRAF-positive gliomas. Early results from this trial were promising, with a significant proportion of participants experiencing tumor shrinkage or stable disease.
Building on the success of FIREFLY-1, the FIREFLY-2 study is a pivotal Phase 3 trial that aims to establish tovorafenib as a first-line therapy for newly diagnosed pediatric low-grade gliomas. This trial is actively recruiting participants and is expected to provide more definitive evidence of the drug’s efficacy compared to standard chemotherapy treatments.
Preliminary data from these trials suggest that tovorafenib can achieve tumor shrinkage in a substantial number of patients, with some experiencing complete remission of their tumors. This is particularly important given that current treatment options for pLGG can sometimes fail, and new therapies are desperately needed.
Side effects observed in the trials were generally manageable, with most patients experiencing mild issues like skin rashes or changes in hair color. Serious side effects, such as liver toxicity or growth delay, were rare but did occur in a small number of patients, highlighting the importance of close monitoring during treatment.
Side Effects and Considerations
While tovorafenib is generally well-tolerated, like any drug, it comes with potential side effects. The most commonly reported side effects are hair color changes, skin rashes, and anemia. These side effects are usually mild and reversible once the treatment is completed.
However, more serious side effects can occur. Some children experienced liver issues, such as elevated liver enzymes, which requires regular monitoring of liver function during treatment. Additionally, since tovorafenib targets specific genetic mutations in tumor cells, there is a possibility of growth delays in pediatric patients, although this has been relatively rare.
Because tovorafenib is still undergoing clinical trials, its long-term safety profile is not yet fully established. That being said, early results indicate that the benefits of the drug outweigh the risks for many children with pLGG, especially when compared to traditional chemotherapy treatments that often come with more severe side effects.
Why Tovorafenib Matters
Tovorafenib represents a significant advancement in the treatment of pediatric low-grade gliomas. It has received Breakthrough Therapy designation from the FDA, acknowledging its potential to offer substantial improvements over existing treatments. This designation accelerates the drug’s development and regulatory review, allowing it to reach patients sooner.
For families dealing with a diagnosis of pLGG, tovorafenib offers hope where traditional therapies may have fallen short. Its ability to target the underlying genetic mutations in these tumors means that treatment is more personalized and potentially more effective. This shift toward precision medicine could change the way pediatric brain tumors are treated in the future.
Furthermore, the drug’s success in clinical trials could open the door for its use in other cancers that involve similar genetic mutations, broadening its impact beyond pediatric gliomas.
Future of Tovorafenib
The future of tovorafenib is promising. With ongoing studies like FIREFLY-2, researchers hope to confirm its role as a first-line treatment for pediatric low-grade gliomas. If these trials are successful, tovorafenib could be approved for widespread use in treating these tumors, offering a much-needed alternative to chemotherapy.
In addition, as targeted therapies continue to evolve, tovorafenib may serve as a model for future cancer treatments, demonstrating the power of precision medicine in improving outcomes for patients with complex genetic conditions.
Conclusion
Tovorafenib is a groundbreaking drug that offers new hope for children diagnosed with pediatric low-grade gliomas. Its ability to target specific genetic mutations in these tumors, along with its manageable side effect profile, makes it a promising alternative to traditional chemotherapy. As clinical trials continue and further results emerge, tovorafenib could reshape the treatment landscape for pediatric brain tumors, providing more effective and less toxic options for children in need.
While there’s still much to learn about its long-term effects, the early success of tovorafenib is a testament to the potential of targeted therapies in the fight against cancer. For families facing the difficult journey of pediatric brain cancer, tovorafenib represents a beacon of hope for a brighter future.
References
- Dhillon S. (2024). Tovorafenib: First Approval. Drugs, 84(8), 985-993. DOI
- Kilburn LB, Khuong-Quang DA, Hansford JR, et al. (2024). The type II RAF inhibitor tovorafenib in relapsed/refractory pediatric low-grade glioma: the phase 2 FIREFLY-1 trial. Nat Med, 30(1), 207-217. DOI
- Yaman I, Bouffet E. (2024). How will tovorafenib change our treatment of pediatric low-grade glioma? Expert Opin Emerg Drugs, 29(1), 1-3. DOI
- Sidaway P. (2024). Tovorafenib effective against low-grade gliomas harbouring BRAF fusions. Nat Rev Clin Oncol, 21(2), 83. DOI
- Zhang T, Xu B, Tang F, et al. (2024). Type II RAF inhibitor tovorafenib for the treatment of pediatric low-grade glioma. Expert Rev Clin Pharmacol, 17(11), 999-1008. DOI
- Khoury JE, Wehbe S, Attieh F, et al. (2024). A critical review of RAF inhibitors in BRAF-mutated glioma treatment. Pharmacogenomics, 25(7), 343-355. DOI