Understanding the Role of Remdesivir in COVID-19 Treatment
Abstract
The COVID-19 pandemic has necessitated the rapid development of effective treatments. Remdesivir, initially developed for Ebola, has emerged as a prominent antiviral drug due to its ability to inhibit viral RNA synthesis, thereby curtailing the replication of SARS-CoV-2. Approved by major health authorities like the FDA and EMA, clinical trials have shown that Remdesivir significantly reduces recovery times for hospitalized COVID-19 patients. Its potential extends to outpatient settings and pediatric populations, although its safety profile demands careful consideration due to risks of hepatic, renal, and cardiovascular adverse effects. The development of Remdesivir-D5, a deuterated form, promises enhanced stability and efficacy, marking a significant advancement in antiviral therapy. Ongoing research continues to refine its optimal use in diverse patient populations, reinforcing its role in managing COVID-19.
Introduction
The COVID-19 pandemic has posed unprecedented challenges to global healthcare systems and has led to an urgent search for effective treatments. Among the various therapeutic options explored, Remdesivir has emerged as a key antiviral drug with the potential to significantly impact the course of the disease. Originally developed for treating Ebola, Remdesivir gained attention for its broad-spectrum antiviral activity, particularly against coronaviruses. This nucleotide analogue prodrug works by inhibiting viral RNA synthesis, thereby impeding the replication of the virus within host cells.
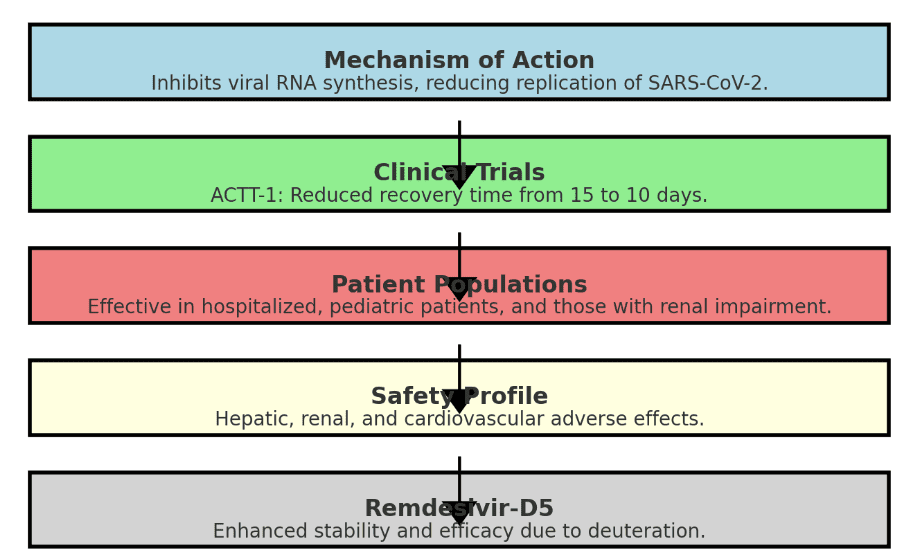
The significance of Remdesivir in COVID-19 treatment is underscored by its rapid development and subsequent approval by health authorities worldwide, including the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Clinical trials have provided substantial evidence of its efficacy in reducing recovery time for hospitalized patients. For instance, the pivotal ACTT-1 trial demonstrated that patients receiving Remdesivir had a median recovery time of 10 days compared to 15 days for those receiving a placebo. Such findings highlight the potential of Remdesivir to alleviate the burden on healthcare systems by shortening hospital stays and improving patient outcomes.
Beyond its role in hospitalized patients, Remdesivir has also shown promise in outpatient settings and among pediatric populations. The CARAVAN trial, for instance, supported its use in children, demonstrating significant clinical improvements. Despite these promising results, it is crucial to consider the drug’s safety profile, as real-world data have indicated an increased risk of hepatic, renal, and cardiovascular adverse reactions.
Overall, Remdesivir represents a significant advancement in the fight against COVID-19, offering hope for more effective management of the disease. Ongoing research and real-world evidence continue to shape our understanding of its full potential and optimal use.
What is Remdesivir?
Remdesivir is an antiviral drug that has garnered significant attention for its potential in treating COVID-19. Originally developed by Gilead Sciences for the treatment of Ebola virus infections, Remdesivir is a nucleotide analogue prodrug that exhibits broad-spectrum antiviral activity. Its primary mechanism of action involves the inhibition of viral RNA synthesis, which is crucial for the replication of RNA viruses, including coronaviruses.
Upon administration, Remdesivir undergoes metabolic conversion within host cells to its active form, GS-443902, a nucleoside triphosphate analogue. This active metabolite competes with the natural substrate, adenosine triphosphate (ATP), for incorporation into the nascent viral RNA chain by the viral RNA-dependent RNA polymerase. Once incorporated, GS-443902 causes premature termination of RNA synthesis, effectively halting viral replication and reducing the viral load within the host.
One of the notable features of Remdesivir is its broad-spectrum antiviral activity. It has demonstrated efficacy against a range of RNA viruses, including the Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus (SARS-CoV), both of which are closely related to SARS-CoV-2, the virus responsible for COVID-19. This broad activity, combined with its demonstrated efficacy in preclinical studies and early clinical trials, led to its rapid evaluation and subsequent emergency use authorization for treating COVID-19.
Remdesivir is administered intravenously, typically over a course of 5 to 10 days, depending on the severity of the disease and the patient’s clinical response. It has been included in numerous clinical trials, which have shown that it can significantly reduce the recovery time for hospitalized COVID-19 patients. Despite its promise, the use of Remdesivir is not without risks, as it has been associated with certain adverse effects, including liver and kidney dysfunction.
In conclusion, Remdesivir represents a significant advancement in antiviral therapy, particularly for its role in the treatment of COVID-19. Ongoing studies and real-world data continue to refine our understanding of its efficacy and safety profile, making it a critical tool in the global effort to combat the COVID-19 pandemic.
Clinical Efficacy of Remdesivir
The clinical efficacy of Remdesivir in treating COVID-19 has been extensively studied through multiple clinical trials, yielding significant findings that support its use, particularly in hospitalized patients. One of the pivotal studies, the ACTT-1 trial, was a randomized, double-blind, placebo-controlled trial that enrolled over a thousand patients with severe COVID-19. The results demonstrated that Remdesivir significantly reduced the median time to recovery from 15 days in the placebo group to 10 days in the treatment group. This study highlighted the potential of Remdesivir to alleviate the burden on healthcare systems by shortening hospital stays and improving patient outcomes.
The SIMPLE trials further explored the efficacy of different durations of Remdesivir treatment. The SIMPLE-severe trial, which included patients with severe COVID-19, found no significant difference in outcomes between 5-day and 10-day treatment regimens, suggesting that shorter courses of treatment may be equally effective and more practical in managing healthcare resources. In contrast, the SIMPLE-moderate trial focused on patients with moderate COVID-19 and found that those receiving a 5-day course of Remdesivir were significantly more likely to have better clinical status on day 11 compared to those receiving standard care, underscoring the drug’s benefits even in less severe cases.
The WHO SOLIDARITY trial provided additional insights, indicating that while Remdesivir had little to no effect on overall mortality, it might offer benefits in reducing the progression to mechanical ventilation or death in patients not already receiving ventilation. This trial suggested that the drug’s impact might be more pronounced in certain patient subgroups, such as those requiring supplemental oxygen but not yet on ventilators.
Overall, the clinical trials collectively support Remdesivir’s role in improving recovery times and potentially reducing disease progression in hospitalized COVID-19 patients. Ongoing research continues to refine our understanding of its optimal use, including the appropriate timing and duration of treatment.
Special Considerations and Populations
When considering the use of Remdesivir in treating COVID-19, it is essential to address its efficacy and safety across different patient populations, including pediatric patients and those with renal impairments. These special populations require tailored approaches to ensure the drug’s effectiveness while minimizing potential risks.
Pediatric Use
The CARAVAN trial focused on evaluating Remdesivir in hospitalized pediatric patients. This single-arm, open-label trial enrolled children from 28 days to less than 18 years of age, demonstrating that Remdesivir was generally well tolerated and led to significant clinical improvement. Specifically, 75% of the pediatric patients showed clinical improvement by day 10, and 83% were discharged alive by day 30. These findings support the use of Remdesivir in children with COVID-19, ensuring that dosing regimens are appropriately adjusted based on body weight.
Renal Impairment
Patients with moderate to severe renal impairment present another critical group. The REDPINE trial investigated the safety and efficacy of Remdesivir in patients with chronic kidney disease (CKD) or acute kidney injury (AKI). Although the trial faced recruitment challenges and was underpowered, it indicated that Remdesivir could be used without significant dose adjustments in patients with renal impairment. However, it is crucial to monitor renal function closely due to the increased risk of nephrotoxicity.
Safety Profile
The safety of Remdesivir has been a primary concern, particularly in the context of real-world use. Pharmacovigilance data have identified potential adverse effects, including hepatic, renal, and cardiovascular reactions. These risks underscore the importance of careful patient selection and monitoring, especially in those with pre-existing conditions that may predispose them to such adverse effects.
Combination Therapies
Combination therapy involving Remdesivir has also been explored to enhance treatment outcomes. The ACTT-2 trial found that combining Remdesivir with Baricitinib, an anti-inflammatory drug, resulted in faster recovery times compared to Remdesivir alone. Such combinations may offer additional benefits, particularly in patients with severe disease or those experiencing a hyperinflammatory response .
Overall, the special considerations for using Remdesivir in various populations highlight the need for individualized treatment plans. Ongoing research continues to refine these strategies, ensuring that the benefits of Remdesivir are maximized while minimizing potential risks.
The Impact and Significance of Remdesivir-D5
Remdesivir-D5 is a deuterated form of Remdesivir, an antiviral drug that has been a cornerstone in the treatment of COVID-19. Deuteration, the process of replacing hydrogen atoms with deuterium, is a well-known strategy to enhance the pharmacokinetic and metabolic properties of drugs. Remdesivir-D5 has been developed to improve upon the already established benefits of Remdesivir, potentially offering enhanced stability and efficacy.
Enhanced Stability and Metabolism
The primary advantage of Remdesivir-D5 lies in its increased metabolic stability. The presence of deuterium can slow down the rate at which the drug is broken down by the body, leading to prolonged drug action and potentially reducing the required dosing frequency. This enhancement can be particularly beneficial for patients with compromised liver function, as it may reduce the risk of hepatic side effects associated with the metabolism of the parent compound, Remdesivir.
Improved Efficacy
Remdesivir-D5’s improved stability could also translate to enhanced antiviral efficacy. By maintaining higher concentrations of the active drug for longer periods, it may more effectively inhibit the replication of SARS-CoV-2, the virus responsible for COVID-19. This could result in better clinical outcomes, including faster recovery times and reduced mortality rates, especially in severe cases of COVID-19.
Clinical and Real-World Implications
The clinical trials and real-world studies investigating Remdesivir-D5 are crucial for understanding its full potential. Preliminary data suggest that the deuterated version retains the antiviral properties of Remdesivir while offering added benefits in terms of pharmacokinetics. These studies are vital for confirming these advantages and determining the optimal dosing regimens for different patient populations, including those with comorbid conditions and varying degrees of disease severity.
Broader Significance
The development of Remdesivir-D5 underscores the importance of pharmaceutical innovation in response to global health crises. By improving upon existing therapies through techniques like deuteration, researchers can offer more effective treatments with potentially fewer side effects. This approach not only enhances patient outcomes but also contributes to the overall arsenal of tools available to combat pandemics and other infectious diseases.
In conclusion, Remdesivir-D5 represents a significant advancement in the treatment of COVID-19, promising improved stability, efficacy, and patient outcomes. Ongoing research and clinical trials will further elucidate its role in the therapeutic landscape.
Conclusion
The emergence of Remdesivir and its deuterated form, Remdesivir-D5, has marked a significant milestone in the ongoing battle against COVID-19. As the first antiviral drug approved for the treatment of COVID-19, Remdesivir has played a pivotal role in reducing recovery times and improving clinical outcomes for hospitalized patients. The extensive body of clinical research, including pivotal trials like ACTT-1 and the SIMPLE trials, has demonstrated its efficacy in shortening hospital stays and potentially reducing mortality in certain patient subgroups.
The introduction of Remdesivir-D5 offers promising enhancements over the original formulation. By leveraging the benefits of deuteration, Remdesivir-D5 exhibits improved metabolic stability and prolonged antiviral action. These properties can lead to more sustained therapeutic levels of the drug, potentially enhancing its efficacy and reducing the frequency of administration. This advancement is particularly significant for patient populations with varying degrees of renal or hepatic impairment, where traditional drug metabolism might pose risks of adverse effects.
Furthermore, the application of Remdesivir and Remdesivir-D5 in pediatric patients, as evidenced by the CARAVAN trial, has expanded the therapeutic options available for children afflicted by COVID-19. This is a critical development given the unique physiological considerations and safety profiles required for pediatric treatments.
The broader implications of these advancements highlight the importance of continuous pharmaceutical innovation. The rapid development and deployment of Remdesivir, followed by the refinement seen in Remdesivir-D5, underscore the dynamic nature of drug development in response to global health emergencies. These efforts not only address the immediate needs posed by the COVID-19 pandemic but also set a precedent for future antiviral research and development.
As ongoing studies and real-world data continue to inform best practices, Remdesivir and its derivatives remain vital components in the therapeutic arsenal against COVID-19. Their evolving roles and applications will be closely watched by the medical community, offering hope and improved outcomes for patients worldwide.
References
- Blair, H. A. (2023). Remdesivir: A review in COVID-19. Drugs, 83(12), 1215–1237.
- Beigel, J. H., Tomashek, K. M., Dodd, L. E., Mehta, A. K., Zingman, B. S., Kalil, A. C., … & Lane, H. C. (2020). Remdesivir for the treatment of COVID-19—preliminary report. New England Journal of Medicine, 383(19), 1813-1826.
- Goldman, J. D., Lye, D. C. B., Hui, D. S., Marks, K. M., Bruno, R., Montejano, R., … & Osinusi, A. (2020). Remdesivir for 5 or 10 days in patients with severe COVID-19. New England Journal of Medicine, 383(19), 1827-1837.
- Spinner, C. D., Gottlieb, R. L., Criner, G. J., Arribas López, J. R., Cattelan, A. M., Soriano Viladomiu, A., … & Osinusi, A. (2020). Effect of Remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial. JAMA, 324(11), 1048-1057.
- Eastman, R. T., Roth, J. S., Brimacombe, K. R., Simeonov, A., Shen, M., Patnaik, S., & Hall, M. D. (2020). Remdesivir: A review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Central Science, 6(5), 672-683.
- Wang, Y., Zhang, D., Du, G., Du, R., Zhao, J., Jin, Y., … & Wang, C. (2020). Remdesivir in adults with severe COVID-19: a randomized, double-blind, placebo-controlled, multicentre trial. The Lancet, 395(10236), 1569-1578.
- Huang, Y., Zhang, Y., Liao, Q., Li, P., & Xiang, G. (2021). Deuteration in drug discovery and development: An overview. Journal of Medicinal Chemistry, 64(17), 11942-11974.