Unlocking Health Through Metabolism: How Amino Acids Are Shaping the Future of Medicine
Abstract
Amino acids are no longer viewed merely as protein building blocks—they are now recognized as powerful regulators of human health and disease. Recent research has uncovered their vital roles in cancer proliferation, metabolic disorders like diabetes and obesity, immune cell activation, and neurological development. This blog explores how tumors hijack amino acid metabolism for growth, how imbalances contribute to insulin resistance and heart disease, and how targeting these pathways is paving the way for novel therapies. With clinical applications ranging from glutaminase inhibitors to dietary modulation, amino acid metabolism is emerging as a central focus of precision medicine.
Introduction — Why Amino Acid Metabolism Matters More Than Ever
Amino acids have long been recognized as the fundamental building blocks of proteins, but modern research reveals a much broader and more dynamic role. Today, amino acid metabolism is emerging as a central player in a wide spectrum of biological functions—ranging from cellular energy production and immune regulation to signaling cascades and disease progression. This shift in perspective is transforming our understanding of health and disease, making amino acid metabolism a hotbed of scientific discovery and therapeutic innovation.
Far from being passive nutrients, amino acids actively participate in key regulatory pathways such as the mammalian target of rapamycin (mTOR) signaling, oxidative stress response, and immune cell activation. Their transport and availability are tightly controlled by specialized amino acid transporters (AATs), such as members of the solute carrier (SLC) family, which function not only as conduits but also as sensors and modulators of nutrient status within cells.
Perhaps most compelling is the mounting evidence linking amino acid metabolism to disease. In cancer, for instance, tumor cells exhibit a voracious appetite for amino acids like glutamine and branched-chain amino acids (BCAAs), using them to fuel uncontrolled proliferation. In metabolic diseases like type 2 diabetes and obesity, altered BCAA levels have been shown to disrupt insulin sensitivity. Even in immune and neurological disorders, amino acids influence cell differentiation, signal transduction, and neurotransmitter synthesis.
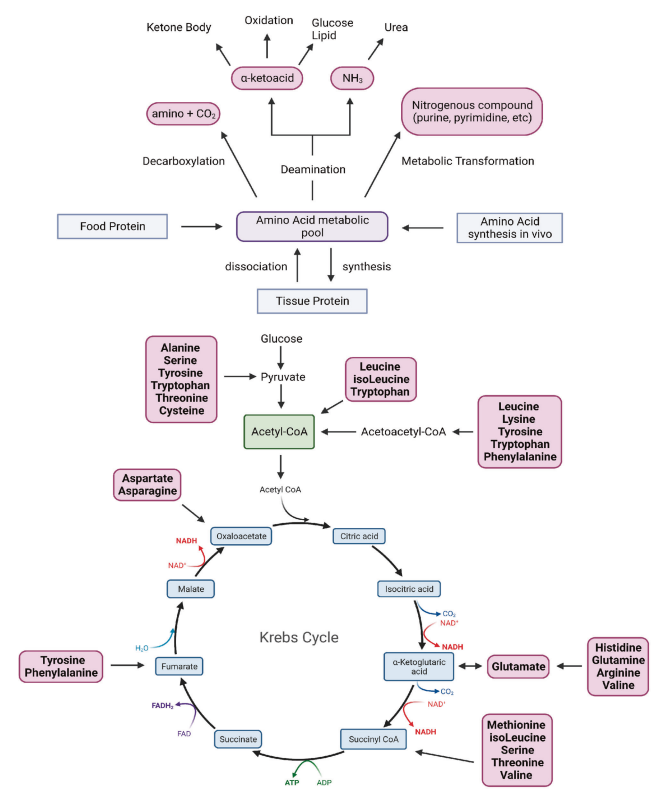
Fig. 1 Overview of amino acid metabolism.
These discoveries have sparked a new wave of interest in targeting amino acid metabolism for therapy. From inhibitors of glutaminase in cancer to dietary interventions in metabolic syndrome, the therapeutic potential is vast and still unfolding.
Understanding amino acid metabolism isn’t just an academic pursuit—it’s the foundation of a new frontier in medicine. As we unlock the intricate web of amino acid regulation, we inch closer to novel diagnostics and personalized treatments that could reshape patient care across disciplines.
The Tumor Connection — How Cancer Cells Hijack Amino Acid Metabolism
Cancer cells are notorious for their metabolic adaptations, and among the most critical of these is their dependence on amino acids. Unlike normal cells, tumors often exhibit what is known as “amino acid addiction”—a metabolic rewiring that allows them to sustain rapid proliferation, evade apoptosis, and manipulate their surrounding microenvironment.
One of the most prominent examples is glutamine metabolism. Glutamine serves as a carbon and nitrogen donor, fueling the tricarboxylic acid (TCA) cycle and supporting nucleotide, lipid, and amino acid biosynthesis. Many tumors overexpress glutamine transporters like SLC1A5 (ASCT2) and activate glutaminase (GLS) to convert glutamine into glutamate and α-ketoglutarate, thereby maintaining high levels of energy and biosynthetic precursors. This glutamine-driven pathway has made GLS inhibitors like CB-839 a promising therapeutic strategy in cancers such as triple-negative breast cancer and renal cell carcinoma.
Equally important are branched-chain amino acids (BCAAs)—leucine, isoleucine, and valine—which activate the mTORC1 pathway, a central regulator of protein synthesis and cell growth. Tumor types such as pancreatic ductal adenocarcinoma (PDAC), non-small cell lung cancer (NSCLC), and glioblastoma (GBM) exhibit altered BCAA catabolism. Enzymes like BCAT1/2 and regulators like BCKDK are frequently upregulated or dysregulated, contributing to oncogenic signaling and resistance to therapy.
Emerging evidence also shows that cancer-associated fibroblasts (CAFs) within the tumor microenvironment metabolize and secrete amino acid derivatives such as branched-chain α-keto acids (BCKAs), which cancer cells then repurpose. This metabolic cross-talk highlights the complexity and adaptability of tumor amino acid metabolism.
These insights point toward a powerful therapeutic paradigm: targeting the unique amino acid requirements of cancer cells while sparing normal tissues. As we deepen our understanding of amino acid transporters, enzymes, and their regulation in specific tumor subtypes, the opportunity to develop precision metabolic therapies becomes increasingly tangible.
Beyond Cancer — Amino Acids in Diabetes, Obesity, and Heart Disease
While amino acid metabolism has garnered significant attention in cancer research, its role in metabolic and cardiovascular diseases is equally profound—and increasingly recognized as a potential therapeutic target. One of the most consistent findings in recent years is the link between elevated branched-chain amino acids (BCAAs) and the development of insulin resistance and type 2 diabetes (T2D).
BCAAs—leucine, isoleucine, and valine—are essential amino acids commonly found in high-protein diets. Studies have shown that elevated plasma levels of BCAAs correlate with insulin resistance and predict future development of T2D. The mechanism behind this involves impaired BCAA catabolism in tissues like liver, muscle, and adipose tissue, leading to the accumulation of toxic BCAA-derived metabolites that interfere with insulin signaling pathways. A pivotal enzymatic regulator in this process is BCKDH (branched-chain α-keto acid dehydrogenase), which becomes inactivated when its kinase, BCKDK, is upregulated—an event commonly seen in obesity.
Interestingly, dietary interventions targeting specific BCAAs have yielded promising results. A low-isoleucine diet, for example, has been shown to improve insulin sensitivity and increase energy expenditure in obese mouse models. This highlights the nuanced, amino acid-specific effects within the BCAA group and supports the idea of precision nutrition in metabolic disease management.
In cardiovascular health, amino acids also play a dual role. For instance, glutamine supports antioxidant defense in cardiomyocytes by fueling the production of glutathione (GSH), a crucial molecule in oxidative stress protection. Moreover, altered amino acid metabolism is associated with cardiac hypertrophy, heart failure, and impaired mitochondrial function in conditions like pulmonary hypertension.
Collectively, these insights underscore that amino acid metabolism is not confined to the realm of oncology but is equally vital in regulating systemic metabolism and cardiovascular function. By targeting specific enzymes or modifying dietary intake, researchers are paving the way for novel therapeutic strategies to combat metabolic syndrome, obesity, and heart disease.
Immune and Neurological Insights — More Than Metabolism
Amino acids are far more than metabolic fuels or structural components of proteins—they are indispensable regulators of immune and neurological function. Recent research highlights how amino acid metabolism shapes the fate, function, and survival of immune cells, particularly T lymphocytes, and plays a central role in maintaining brain health and development.
In the immune system, amino acids such as arginine, tryptophan, leucine, and glutamine regulate T cell proliferation, activation, and differentiation. Upon activation, CD8+ T cells significantly increase the expression of amino acid transporters, such as SLC7A5, to meet heightened metabolic demands. Deficiency in key amino acids like tryptophan or arginine can halt the cell cycle, preventing T cells from entering the S phase and triggering cell exhaustion or death. Furthermore, asparagine (Asn) has been shown to enhance T cell receptor (TCR) signaling by binding to the tyrosine kinase LCK, a crucial step in T cell activation and cytokine production. These insights are not only important for understanding immune responses but also open new avenues for immunotherapy in cancer and autoimmune disease.
In the nervous system, amino acid metabolism is equally critical. One notable example is asparagine synthetase deficiency (ASD), a rare but severe neurological condition caused by mutations in the ASNS gene. This leads to impaired brain development, resulting in intellectual disability, seizures, and atrophy. Aspartate and glutamine are also essential for neurotransmitter synthesis and energy metabolism in neurons. Moreover, emerging studies suggest that D-aspartate (D-Asp), an enantiomer of aspartate, acts as an agonist at NMDA receptors, with implications for psychiatric conditions like schizophrenia. Animal studies have shown that modulating D-Asp levels can impact synaptic plasticity and behavioral outcomes.
Together, these findings emphasize the integrative role of amino acid metabolism across immune and neural tissues. Targeting these pathways may hold therapeutic promise for treating inflammatory diseases, immune deficiencies, and neurodevelopmental disorders.
Therapeutic Horizons — Targeting Metabolism for Next-Gen Medicine
The growing understanding of amino acid metabolism in disease has sparked a wave of interest in developing targeted metabolic therapies. As researchers decode how cancer cells, immune cells, and neurons uniquely utilize amino acids, the opportunity to exploit these dependencies for therapeutic benefit is rapidly unfolding.
In oncology, metabolic vulnerabilities have become actionable targets. One of the most advanced examples is the glutaminase inhibitor CB-839 (telaglenastat), which disrupts glutamine metabolism—an essential nutrient for many tumors. By inhibiting glutaminase (GLS1), CB-839 starves cancer cells of the building blocks needed for growth and survival. Clinical trials have demonstrated promising activity when combined with standard therapies, particularly in renal cell carcinoma and triple-negative breast cancer.
Another area of success lies in arginine-depleting therapies. Certain cancers, such as glioblastoma and hepatocellular carcinoma, are auxotrophic for arginine due to silencing of the ASS1 gene. ADI-PEG20, a pegylated arginine deiminase, exploits this weakness by depleting extracellular arginine, effectively halting tumor proliferation. This precision approach underscores the power of metabolic targeting in personalized cancer care.
Beyond cancer, enzyme therapies like asparaginase have long been used in acute lymphoblastic leukemia (ALL) to deplete asparagine, capitalizing on leukemic cells’ limited capacity for its synthesis. Novel derivatives with improved safety and efficacy profiles are now in development.
Importantly, the therapeutic potential of amino acid metabolism extends to autoimmune, cardiovascular, and metabolic disorders. Emerging research suggests that manipulating BCAA metabolism can improve insulin sensitivity, while modulating amino acid availability in the immune microenvironment could enhance T cell-based immunotherapies.
As precision medicine continues to evolve, targeting amino acid metabolism offers a compelling strategy—one that not only disrupts disease progression but also allows for selective, tissue-specific intervention with fewer side effects. This metabolic lens is redefining how we understand and treat complex diseases across disciplines.
References
Ling, Z. N., Jiang, Y. F., Ru, J. N., Lu, J. H., Ding, B., & Wu, J. (2023). Amino acid metabolism in health and disease. Signal Transduction and Targeted Therapy, 8(1), 345.
https://doi.org/10.1038/s41392-023-01569-3
Brosnan, J. T. (2003). Interorgan amino acid transport and its regulation. The Journal of Nutrition, 133(6), 2068S–2072S.
https://doi.org/10.1093/jn/133.6.2068S
Locasale, J. W. (2013). Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nature Reviews Cancer, 13(8), 572–583.
https://doi.org/10.1038/nrc3557
Tibbitt, C. A., Stark, J. M., Martens, L., Ma, J., Mold, J. E., Deswarte, K., … & Bending, D. (2019). Single-cell RNA sequencing of the T helper cell response to house dust mites defines a distinct gene expression signature in airway memory T cells. Immunity, 51(1), 169–184.e5.
https://doi.org/10.1016/j.immuni.2019.05.012
Altman, B. J., Stine, Z. E., & Dang, C. V. (2016). From Krebs to clinic: Glutamine metabolism to cancer therapy. Nature Reviews Cancer, 16(10), 619–634.
https://doi.org/10.1038/nrc.2016.71
Hattori, A., Tsunoda, M., Konuma, T., Kobayashi, M., Nagy, T., Glushka, J., … & Ito, T. (2017). Cancer progression by reprogrammed BCAA metabolism in myeloid leukemia. Nature, 545(7655), 500–504.
https://doi.org/10.1038/nature22314
Wise, D. R., & Thompson, C. B. (2010). Glutamine addiction: A new therapeutic target in cancer. Trends in Biochemical Sciences, 35(8), 427–433.
https://doi.org/10.1016/j.tibs.2010.05.003
White, P. J., Lapworth, A. L., An, J., Wang, L., McGarrah, R. W., Stevens, R. D., … & Newgard, C. B. (2016). Muscle-specific knockout of branched-chain aminotransferase impairs amino acid metabolism and leads to lean body mass loss. Cell Metabolism, 23(6), 1207–1221.
https://doi.org/10.1016/j.cmet.2016.04.008
Yu, D., Richardson, N. E., Green, C. L., Spicer, A. B., Murphy, C. J., Flores, V., … & Lamming, D. W. (2021). The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metabolism, 33(5), 905–922.e6.
https://doi.org/10.1016/j.cmet.2021.02.018
Wu, D., Sanin, D. E., Everts, B., Chen, Q., Qiu, J., Buck, M. D., … & Pearce, E. J. (2021). The amino acid asparagine supports T cell receptor signaling and aerobic glycolysis. Nature Cell Biology, 23(6), 653–664.
https://doi.org/10.1038/s41556-021-00685-z
Gross, M. I., Demo, S. D., Dennison, J. B., Chen, L., Chernov-Rogan, T., Goyal, B., … & Janes, J. R. (2014). Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Molecular Cancer Therapeutics, 13(4), 890–901.
https://doi.org/10.1158/1535-7163.MCT-13-0793
Delage, B., Fennell, D. A., Nicholson, L., McNeish, I., Lemoine, N. R., Crook, T., & Szlosarek, P. W. (2010). Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. International Journal of Cancer, 126(12), 2762–2772.
https://doi.org/10.1002/ijc.25170