Unlocking Neurotherapeutics: The Promise of Cdk5 Inhibitor 25–106 in Treating Neuropsychiatric Disorders
Abstract
The development of cyclin-dependent kinase 5 (Cdk5) inhibitors, particularly the novel compound 25–106, represents a promising avenue for therapeutic intervention in neuropsychiatric and neurodegenerative disorders. Cdk5 plays a crucial role in neuronal function and is implicated in diseases such as Alzheimer’s and Parkinson’s. Research into 25–106 highlights its potent inhibition of Cdk5 along with its ability to penetrate the blood-brain barrier, suggesting significant therapeutic potential. Future research will focus on refining the pharmacological profiles of Cdk5 inhibitors to enhance specificity and efficacy while exploring innovative drug delivery systems. Understanding the long-term effects of Cdk5 inhibition will be vital to establishing safe and effective treatment options.
Introduction to Cdk5 and Its Role in the Brain
Cyclin-dependent kinase 5 (Cdk5) is a proline-directed kinase predominantly expressed in mature neurons. Unlike traditional cyclin kinases, Cdk5 does not require coactivation by binding to a cyclin partner. Instead, Cdk5 is constitutively activated through interactions with its unique coactivators p35 or p39. Over 3 decades of research on Cdk5 functionality has revealed this kinase to play unique roles in modulating neuronal development, complex behavior, and neurodegeneration. Cdk5 is expressed within excitatory and inhibitory circuitry and regulates glutamatergic, dopaminergic, and GABAergic signaling . The current scenario shows that Cdk5 is a critical regulator of homeostatic neuronal signaling.
Cdk5’s involvement in neuropsychiatric and neurodegenerative diseases has garnered significant interest in the scientific community. Research has shown that dysregulation of Cdk5 is implicated in several neurological disorders, including Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease. In conditions such as Alzheimer’s, Cdk5 can become hyperactivated due to the proteolytic cleavage of its coactivator p35 into p25, leading to aberrant signaling pathways that promote neurodegeneration and neuronal death. This shift from normal Cdk5 function to a neurotoxic enzyme is a hallmark of its role in disease pathology.
In addition to its involvement in neurodegeneration, Cdk5 has also been associated with mood regulation and stress-related behaviors. Studies in animal models have demonstrated that altering Cdk5 activity can affect anxiety, depression, and addiction-related behaviors, further highlighting its significance in neuropsychiatric conditions. Despite the therapeutic potential of targeting Cdk5, developing inhibitors that can effectively modulate its activity in the brain has been challenging. Most Cdk5 inhibitors developed to date struggle with poor brain permeability and non-specificity, limiting their clinical application. Therefore, the search for brain-penetrant, selective Cdk5 inhibitors has become a critical area of research for the treatment of neurological and psychiatric disorders.
Challenges in Developing Cdk5 Inhibitors
The development of effective inhibitors targeting cyclin-dependent kinase 5 (Cdk5) has faced significant challenges that have impeded their clinical application. One of the foremost issues is the kinase’s high degree of homology with other cyclin-dependent kinases (CDKs). This structural similarity results in a lack of specificity among many Cdk5 inhibitors, leading to off-target effects that can complicate therapeutic outcomes. Consequently, inhibitors designed to target Cdk5 often inadvertently affect other kinases, which can result in unwanted side effects and diminish the therapeutic potential of these compounds.
Another critical challenge lies in the ability of Cdk5 inhibitors to penetrate the blood-brain barrier (BBB). Many compounds that show promise in vitro fail to exhibit adequate brain permeability in vivo. This limitation restricts the therapeutic applicability of Cdk5 inhibitors for neurodegenerative and neuropsychiatric diseases, where effective concentrations in the brain are essential for eliciting a pharmacological effect. Traditional methods of drug administration often lead to poor bioavailability and rapid clearance, further complicating the development of viable treatments.
Additionally, the dual role of Cdk5 in both neuroprotection and neurotoxicity adds another layer of complexity to inhibitor development. While inhibition of Cdk5 can be beneficial in the context of neurodegenerative diseases, chronic inhibition may lead to neuronal hyperexcitability and potential seizures, as evidenced by studies on Cdk5 conditional knockout mice. This duality necessitates a careful balance in inhibitor design, aiming to mitigate harmful effects while maximizing therapeutic efficacy.
Lastly, the translational gap from preclinical findings to clinical trials has been a persistent obstacle. Many Cdk5 inhibitors that show promise in animal models do not yield similar results in human studies, highlighting the need for more refined approaches in the design and testing of these compounds.
Introducing the Novel Cdk5 Inhibitor 25–106
The search for effective inhibitors of cyclin-dependent kinase 5 (Cdk5) has led to the promising development of a novel compound, 25–106. This inhibitor has emerged from a series of aminopyrazole-based analogs specifically designed to target Cdk5 while ensuring enhanced brain permeability—a crucial factor for addressing neurological disorders. The unique pharmacological profile of 25–106 offers new hope for therapeutic applications in neuropsychiatric and neurodegenerative diseases, where Cdk5 plays a pivotal role.
One of the standout features of 25–106 is its ability to effectively cross the blood-brain barrier (BBB), a significant barrier for many potential drug candidates. This characteristic is essential for achieving therapeutic concentrations within the central nervous system, where Cdk5 activity is implicated in a variety of conditions, including Alzheimer’s and Parkinson’s diseases. Studies have shown that 25–106 demonstrates high potency in inhibiting Cdk5 both in vitro and in vivo, marking it as a strong candidate for further development.
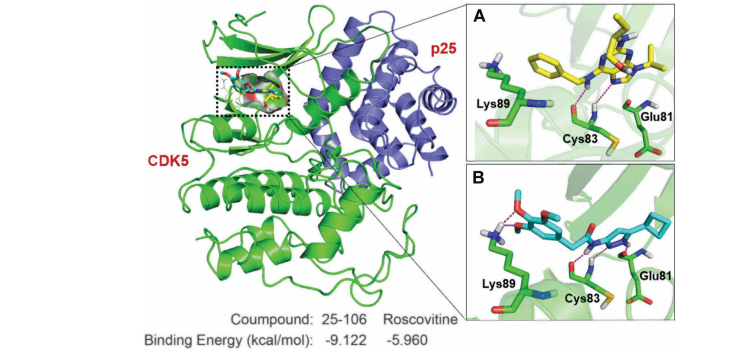
Figure: Key interactions made by 25–106 and roscovitine with CDK5/p25.
In preclinical models, administration of 25–106 has resulted in marked behavioral changes consistent with reduced anxiety and improved cognition, mirroring the effects observed in Cdk5 conditional knockout mice. These findings suggest that the compound not only inhibits Cdk5 effectively but also modulates neurobehavioral phenotypes associated with its activity, reinforcing the therapeutic potential of targeting this kinase.
Moreover, the molecular modeling of 25–106 has provided insights into its binding interactions with Cdk5, indicating a unique mechanism of action that may minimize off-target effects seen with earlier inhibitors. The structural modifications in 25–106 enable it to form additional hydrogen bonds within the kinase’s ATP binding site, resulting in improved specificity and efficacy compared to existing compounds like roscovitine.In summary, 25–106 represents a significant advancement in the development of Cdk5 inhibitors, with promising implications for treating a range of neurological disorders.
Neurobehavioral Effects of 25–106
The novel cyclin-dependent kinase 5 (Cdk5) inhibitor, 25–106, has shown remarkable effects on neurobehavioral responses in preclinical models, particularly in areas related to anxiety, depression, and locomotor activities. Behavioral tests, such as the open-field test and the tail suspension test, were employed to assess the impact of 25–106 on anxiety-like and depressive-like behaviors in mice. These tests are standard methods for evaluating how pharmacological interventions influence animal models of neuropsychiatric conditions.
In the open-field test, which measures anxiety-related behavior, mice treated with 25–106 exhibited significant reductions in anxiety-like symptoms. They spent more time in the center of the open field, a sign of decreased anxiety, and also showed increased locomotion and exploratory behavior. This indicates that 25–106 has anxiolytic effects, which align with previous findings on Cdk5 inhibition and anxiety regulation. These results suggest that 25–106 could be useful in treating anxiety disorders by modulating Cdk5-related pathways in the brain.
Furthermore, the tail suspension test, which is commonly used to evaluate depressive-like behaviors, revealed that 25–106 significantly reduced immobility time in mice. This reduction is indicative of an antidepressant-like effect, as animals treated with the inhibitor showed greater resilience and motivation to escape from stressful conditions. These effects parallel the neurobehavioral changes observed in Cdk5 conditional knockout mice, where reduced Cdk5 activity leads to improved stress responses and reduced depressive symptoms.
The combination of anxiolytic and antidepressant-like effects observed with 25–106 suggests that this compound holds promise for treating mood disorders, particularly those related to stress and anxiety. Importantly, these behavioral outcomes are consistent with Cdk5’s known role in regulating neuronal circuits associated with emotional regulation and stress responses, making 25–106 a promising candidate for further therapeutic development.
Future of Cdk5 Inhibitor Research and Therapeutic Potential
The future of Cdk5 inhibitor research, particularly with compounds like 25–106, holds significant promise for advancing therapeutic strategies in neuropsychiatric and neurodegenerative disorders. Given Cdk5’s critical role in regulating neuronal function and its involvement in various pathologies, targeting this kinase presents an opportunity to develop novel treatments that can address the underlying mechanisms of diseases such as Alzheimer’s, Parkinson’s, and mood disorders.
Research will likely focus on refining the pharmacological profiles of existing inhibitors to enhance their specificity and brain permeability. The discovery of 25–106, which demonstrates both potent inhibition of Cdk5 and effective CNS penetration, sets a precedent for future compound development. Further optimization of its chemical structure could lead to even more effective inhibitors with reduced off-target effects, thereby minimizing potential side effects.
Moreover, the integration of advanced drug delivery systems, such as nanoparticles or conjugated compounds, could improve the bioavailability of Cdk5 inhibitors, ensuring that therapeutic levels are reached in the brain. This approach could potentially overcome the challenges associated with the blood-brain barrier, a critical hurdle in treating central nervous system disorders.
As research continues, it will also be essential to explore the long-term effects of Cdk5 inhibition on neuronal health and function. Understanding the balance between neuroprotection and potential risks associated with chronic inhibition will be vital. Longitudinal studies in preclinical models and subsequent clinical trials will be necessary to evaluate the therapeutic efficacy and safety profiles of new Cdk5 inhibitors.
Ultimately, the therapeutic potential of Cdk5 inhibitors like 25–106 could transform the treatment landscape for various neurological conditions, providing much-needed options for patients suffering from these debilitating disorders.
References
- Dhavan, R., & Tsai, L. H. (2001). A decade of CDK5. Nature Reviews Molecular Cell Biology, 2(10), 749-759.
- Patrick, G. N., Zukerberg, L., Nikolic, M., de la Monte, S., Dikkes, P., & Tsai, L. H. (1999). Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature, 402(6762), 615-622.
- Plattner, F., Hernández, A., Kistler, T. M., Pozo, K., Zhong, P., Yuen, E. Y., & Bibb, J. A. (2015). Memory enhancement by targeting Cdk5 regulation of NR2B. Neuron, 81(5), 1070-1083.
- Bibb, J. A., Snyder, G. L., Nishi, A., Yan, Z., Meijer, L., Fienberg, A. A., & Greengard, P. (1999). Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature, 402(6762), 669-671.
- Cruz, J. C., Tseng, H. C., Goldman, J. A., Shih, H., & Tsai, L. H. (2003). Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron, 40(3), 471-483.
- Sundaram, J. R., Chan, E. S., Poore, C. P., Pareek, T. K., Cheong, W. F., Shui, G., & Sze, S. K. (2013). Specific inhibition of p25/Cdk5 activity by the Cdk5 inhibitory peptide reduces neurodegeneration in vivo. Journal of Neuroscience, 33(1), 334-343.
- Barnett, D. G., & Bibb, J. A. (2011). The role of Cdk5 in cognition and neuropsychiatric and neurological pathology. Brain Research Bulletin, 85(1-2), 9-13.
- Lee, M. S., Kwon, Y. T., Li, M., Peng, J., Friedlander, R. M., & Tsai, L. H. (2000). Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature, 405(6784), 360-364.
- Cicenas, J., Kalyan, K., Sorokinas, A., Stankunas, E., Levy, J., & Meskinyte, I. (2015). Roscovitine in cancer and other diseases. Annals of Translational Medicine, 3(3), 135.
- Khair, N. Z., Lenjisa, J. L., Tadesse, S., Kumarasiri, M., Basnet, S. K. C., & Mekonnen, L. B. (2019). Discovery of CDK5 inhibitors through structure-guided approach. ACS Medicinal Chemistry Letters, 10(6), 786-791.
- Menn, B., Bach, S., Blevins, T. L., Campbell, M., Meijer, L., & Timsit, S. (2010). Delayed treatment with systemic (S)-roscovitine provides neuroprotection and inhibits in vivo CDK5 activity increase in animal stroke models. PLoS One, 5(10), e12117.
- Pao, P. C., & Tsai, L. H. (2021). Three decades of Cdk5. Journal of Biomedical Science, 28(1), 79.
- Rana, S., Sonawane, Y. A., Taylor, M. A., Kizhake, S., Zahid, M., & Natarajan, A. (2018). Synthesis of aminopyrazole analogs and their evaluation as CDK inhibitors for cancer therapy. Bioorganic & Medicinal Chemistry Letters, 28(22), 3736-3740.
- Plattner, F., Hayashi, K., Hernández, A., Benavides, D. R., Tassin, T. C., Tan, C., & Bibb, J. A. (2015). The role of ventral striatal cAMP signaling in stress-induced behaviors. Nature Neuroscience, 18(7), 1094-1100.
- Umfress, A., Singh, S., Ryan, K. J., Chakraborti, A., Plattner, F., Sonawane, Y., Mallareddy, J. R., Acosta, E. P., Natarajan, A., & Bibb, J. A. (2022). Systemic administration of a brain permeable Cdk5 inhibitor alters neurobehavior. Frontiers in Pharmacology, 13, 863762.
- Hawasli, A. H., Benavides, D. R., Nguyen, C., Kansy, J. W., Hayashi, K., Chambon, P., & Bibb, J. A. (2007). Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nature Neuroscience, 10(7), 880-886.
- Yoo, B. C., & Lubec, G. (2001). p25 protein in neurodegeneration. Nature, 411(6835), 763-765.




