Unlocking the Potential of Isotopic Labeling in Pharmaceutical Research
Abstract
Isotopic labeling is a crucial technique in pharmaceutical research, offering detailed insights into the behavior of drugs within biological systems. This method involves incorporating stable or radioactive isotopes into molecules to study their absorption, distribution, metabolism, and excretion (ADME) processes. Key applications include deuterium labeling for improved drug stability and tritium labeling for early-stage drug discovery. Recent advancements in hydrogen isotope exchange (HIE) and late-stage functionalization have enhanced the efficiency and versatility of isotopic labeling. Despite challenges in synthesizing short-lived isotopes and optimizing reaction conditions, future research aims to focus on sustainability and reducing radioactive waste. Collaborative efforts between academia and industry continue to drive innovation, promising significant advancements in drug development and safer, more effective therapeutics.
Keywords: Isotopic Labeling, Drug Discovery, Deuterium Labeling, Hydrogen Isotope Exchange, Radioactive Waste Reduction
Introduction to Isotopic labeling
Isotopic labeling is a powerful and versatile technique used extensively in pharmaceutical research to understand and trace the pathways of drugs within biological systems. This method involves incorporating isotopes—atoms with the same number of protons but different numbers of neutrons—into molecules. These isotopes can be either stable, such as deuterium (²H) and carbon-13 (¹³C), or radioactive, like tritium (³H) and carbon-14 (¹⁴C). By tracking these labeled molecules, scientists gain invaluable insights into the pharmacokinetics and pharmacodynamics of new drug candidates, enabling them to observe how drugs are absorbed, distributed, metabolized, and excreted in living organisms.
The importance of isotopic labeling in pharmaceutical research cannot be overstated. It provides a unique way to study the detailed mechanisms of drug action and metabolism at the molecular level without significantly altering the chemical properties of the compounds. This precision allows for accurate and reliable data, which is crucial for drug development. For instance, deuterium labeling can enhance the stability of a drug, improving its pharmacokinetic properties and making it more effective. Tritium labeling, on the other hand, is essential for early-stage drug discovery, facilitating the study of drug-receptor interactions and binding assays.
This text aims to shed light on the advancements and applications of isotopic labeling in the pharmaceutical industry. Various techniques employed in isotopic labeling will be explored, along with the collaborative efforts driving innovation and the challenges that remain in this field. By understanding these aspects, researchers and professionals can better appreciate the impact of isotopic labeling on drug discovery and development.
What is Isotopic Labeling?
Isotopic labeling is a scientific technique that involves the incorporation of isotopes into molecules to trace and study chemical and biological processes. Isotopes are variants of elements that have the same number of protons but differ in the number of neutrons, resulting in different atomic masses. This variation allows isotopes to be used as markers or tracers in various research applications without significantly altering the chemical properties of the molecules they are integrated into.
Definition and Basic Principles
The fundamental principle of isotopic labeling lies in the ability to distinguish labeled molecules from their non-labeled counterparts using various detection methods. These methods include mass spectrometry, nuclear magnetic resonance (NMR) spectroscopy, and imaging techniques like positron emission tomography (PET). By tracking the labeled isotopes, researchers can gain detailed insights into the behavior, distribution, and interaction of molecules in complex biological systems.
Isotopic labeling can be achieved by incorporating isotopes at specific positions within a molecule. This can be done during the synthesis of the molecule or through isotope exchange reactions where specific atoms in a molecule are replaced with their isotopic counterparts. This labeling process allows for precise tracking and quantification of molecules in metabolic studies, drug development, and environmental monitoring.
Types of Isotopes Used
Isotopic labeling employs both stable and radioactive isotopes, each serving different purposes in research:
Stable Isotopes: These isotopes do not undergo radioactive decay and remain constant over time. Commonly used stable isotopes include:
- Deuterium (²H): A stable isotope of hydrogen, deuterium is used to study metabolic pathways and improve the pharmacokinetic properties of drugs. It is also used in mass spectrometry as an internal standard.
- Carbon-13 (¹³C): This isotope is used in NMR spectroscopy to elucidate molecular structures and study metabolic processes.
- Nitrogen-15 (¹⁵N): Used in protein structure determination and nitrogen cycle studies.
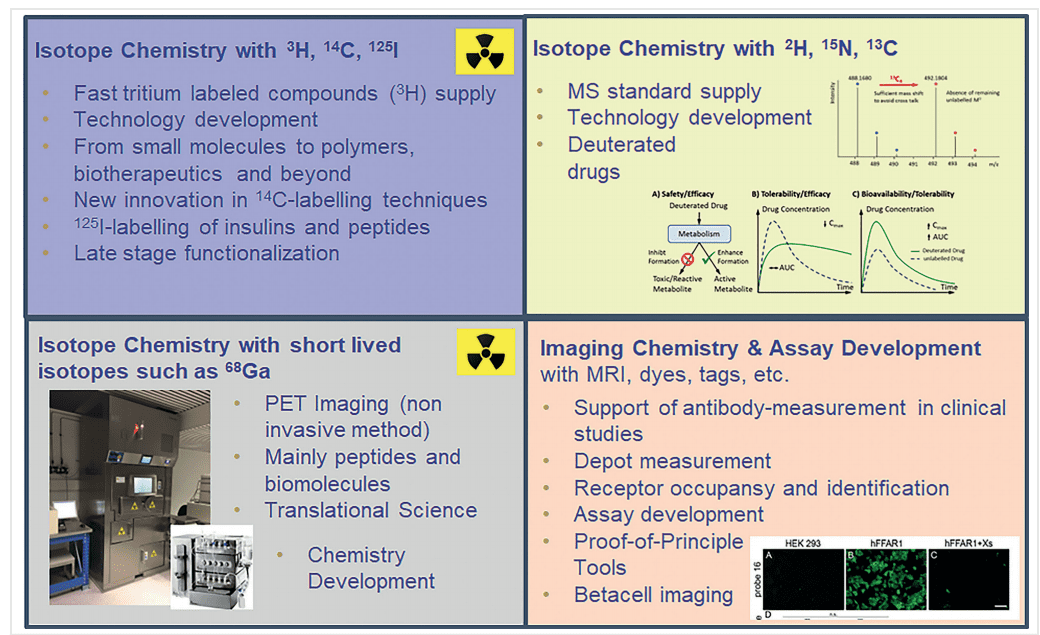
Radioactive Isotopes: These isotopes decay over time, emitting radiation that can be detected using various imaging techniques. They are crucial for tracing and imaging in biological systems. Key radioactive isotopes include:
- Tritium (³H): A radioactive isotope of hydrogen, tritium is widely used in early-stage drug discovery and in vitro studies due to its low energy and long half-life.
- Carbon-14 (¹⁴C): This isotope is commonly used in long-term metabolic studies and radiolabeling of organic compounds for tracking in biological systems.
- Fluorine-18 (¹⁸F): Used in PET imaging, fluorine-18 is valuable for its relatively short half-life and ability to provide high-resolution images.
By utilizing these isotopes, researchers can conduct detailed and precise studies of molecular interactions, providing invaluable information that drives advancements in fields such as drug development, environmental science, and medical diagnostics.
Key Applications in Drug Discovery
Hydrogen Isotope Labeling
Hydrogen isotope labeling, involving isotopes like deuterium (²H) and tritium (³H), is extensively utilized in drug discovery and development due to its 
Deuterium Labeling
Deuterium labeling is a technique where hydrogen atoms in a molecule are replaced with deuterium, a stable isotope of hydrogen. This method is particularly valuable because deuterium has a similar chemical behavior to hydrogen but a greater mass, leading to a lower vibrational frequency in chemical bonds. This results in a phenomenon known as the kinetic isotope effect, where the deuterium-labeled compounds exhibit enhanced stability and resistance to metabolic degradation.
Benefits and Applications
- Improved Drug Stability: By replacing hydrogen with deuterium in drug molecules, researchers can enhance the metabolic stability of the drug. This can lead to improved pharmacokinetic properties, such as increased half-life and reduced frequency of dosing, making the drug more effective and convenient for patients.
- Mass Spectrometry: Deuterium-labeled compounds serve as internal standards in mass spectrometry. Their unique mass signature allows for precise quantification and analysis of drugs in complex biological matrices, aiding in accurate pharmacokinetic and pharmacodynamic studies.
Tritium Labeling
Tritium labeling involves the incorporation of tritium, a radioactive isotope of hydrogen, into drug molecules. Tritium is favored in early drug discovery due to its ability to emit low-energy beta particles, which can be easily detected.
Significance in Early Drug Discovery
- Radioactive Tracers: Tritium-labeled compounds are invaluable in studying drug-receptor interactions, binding assays, and metabolic pathways. The emitted beta particles provide a precise and sensitive method for tracking the distribution and behavior of drugs within biological systems.
- Cost-Effectiveness: Tritium labeling is relatively cost-effective compared to other radioactive isotopes, making it an attractive option for preliminary studies in drug development.
Carbon-14 Labeling
Carbon-14 (¹⁴C) is a radioactive isotope of carbon used in drug development for its stability and ability to provide detailed information on drug metabolism and pharmacokinetics.
Importance in ADME Studies and First-in-Human Experiments
- ADME Studies: Carbon-14 labeling is crucial in absorption, distribution, metabolism, and excretion (ADME) studies. It allows researchers to trace the entire lifecycle of a drug within the body, providing comprehensive data on how the drug is processed and eliminated.
- First-in-Human Experiments: Due to its long half-life and stable radioactive properties, carbon-14 is ideal for use in first-in-human trials. It helps in understanding the pharmacokinetic profile of new drugs, ensuring their safety and efficacy before proceeding to larger clinical trials.
Challenges Associated with Carbon-14 Labeling
- Synthesis Complexity: Incorporating carbon-14 into drug molecules often requires complex and multi-step synthetic procedures. The use of radioactive materials demands specialized facilities and handling protocols, increasing the cost and time involved in the synthesis process.
- Regulatory Compliance: Working with radioactive isotopes like carbon-14 requires stringent regulatory compliance and safety measures to protect researchers and the environment from radiation exposure.
By leveraging hydrogen and carbon isotopes, pharmaceutical researchers can gain deep insights into drug behavior, facilitating the development of safer and more effective therapeutics.
Innovative Methods and Collaborations
Overview of Recent Advancements in Isotopic Labeling Techniques
Recent years have seen significant advancements in isotopic labeling techniques, driven by the need for more efficient, cost-effective, and precise methods in pharmaceutical research. These innovations have enhanced the ability to track and study the behavior of drugs and other compounds within biological systems, paving the way for breakthroughs in drug discovery and development.
Hydrogen Isotope Exchange (HIE)
Hydrogen isotope exchange (HIE) is a method that allows for the incorporation of deuterium (²H) and tritium (³H) into organic molecules through the exchange of hydrogen atoms. This technique has gained prominence due to its versatility and efficiency.
Methods Using Iridium, Ruthenium, and Nanoparticle Catalysts
- Iridium Catalysts: Iridium-based catalysts have proven highly effective in facilitating HIE reactions. These catalysts enable selective and efficient hydrogen-deuterium exchange, which is crucial for producing deuterium-labeled compounds. The use of iridium catalysts has expanded the scope of substrates that can be labeled, including complex pharmaceuticals and biologically active molecules.
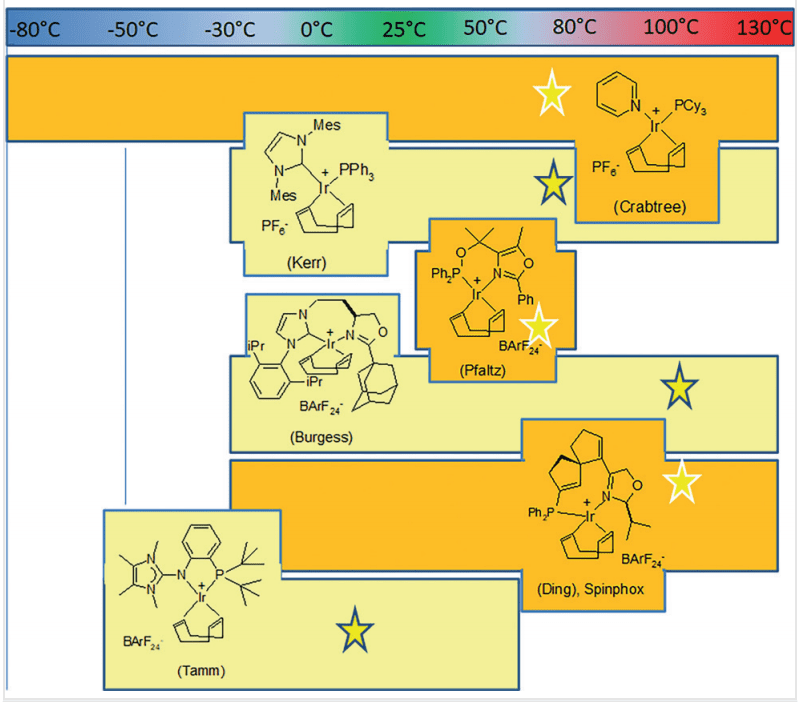
- Ruthenium Catalysts: Ruthenium catalysts offer another powerful approach to HIE. They provide robust catalytic activity and functional group tolerance, allowing for the labeling of a wide range of compounds. Ruthenium-catalyzed HIE is particularly useful for labeling carboxylic acids and other challenging substrates.
- Nanoparticle Catalysts: Recent developments in nanoparticle catalysts have opened new avenues for HIE. These catalysts, composed of metals like iridium or ruthenium, offer high surface area and reactivity. Nanoparticle-catalyzed HIE is characterized by its efficiency and ability to operate under mild conditions, making it an attractive option for labeling sensitive compounds.
Late-Stage Functionalization
Late-stage functionalization refers to the introduction of isotopic labels at the final stages of the synthesis of complex molecules. This approach minimizes the number of radioactive synthetic steps, reducing costs, time, and radioactive waste.
Techniques and Benefits
- Selective Labeling: Late-stage functionalization techniques allow for the selective incorporation of isotopes into specific positions within a molecule. This precision is essential for studying the behavior and interaction of drugs with biological targets.
- Enhanced Efficiency: By incorporating isotopes at the final stages of synthesis, researchers can avoid multiple steps involving radioactive materials, thereby enhancing the overall efficiency and safety of the labeling process.
Collaborative Efforts
Collaboration between academic institutions and industrial partners has been pivotal in advancing isotopic labeling techniques. These collaborations leverage the strengths of both sectors, combining academic innovation with industrial resources and expertise.
Examples of Successful Collaborations
- Academic-Industry Partnerships: Partnerships between universities and pharmaceutical companies have led to the development of new isotopic labeling methodologies and the optimization of existing ones. For example, collaborations have resulted in the creation of novel iridium and ruthenium catalysts for HIE.
- Interdisciplinary Research: Collaborative efforts that bring together chemists, biologists, and pharmacologists have expanded the applications of isotopic labeling. Interdisciplinary research has facilitated the development of labeling techniques that are applicable across various fields, including drug discovery, metabolic studies, and environmental science.
Impact on Advancing Isotope Science
The impact of these collaborative efforts is significant, driving the field of isotope science forward. Enhanced isotopic labeling techniques have led to more accurate and reliable data, improved drug development processes, and a deeper understanding of biological mechanisms. These advancements ultimately contribute to the creation of safer and more effective therapeutics.
Challenges and Future Directions
Current Challenges in Isotopic Labeling
Despite significant advancements in isotopic labeling techniques, several challenges persist that hinder the full potential of these methods in pharmaceutical research. Addressing these challenges is crucial for enhancing the efficiency, safety, and applicability of isotopic labeling.
Synthesis of Short-Lived Isotopes
One of the primary challenges in isotopic labeling is the synthesis and application of short-lived isotopes, such as fluorine-18 (¹⁸F) and gallium-68 (⁶⁸Ga). These isotopes are invaluable for diagnostic imaging techniques like positron emission tomography (PET) due to their ability to provide high-resolution images. However, their short half-lives pose significant difficulties:
- Rapid Decay: The short half-lives of these isotopes require that they be synthesized and utilized within a limited timeframe. This constraint demands highly efficient synthesis processes and immediate application, often necessitating on-site production facilities.
- Logistical Challenges: Transporting short-lived isotopes to remote locations is impractical, limiting their use to facilities with the necessary infrastructure for on-site production and immediate utilization.
Optimizing Reaction Conditions for Higher Yields
Another challenge in isotopic labeling is optimizing reaction conditions to achieve higher yields of labeled compounds. The complexity of these reactions often results in low yields, increasing the cost and time required for producing sufficient quantities of labeled material:
- Catalyst Efficiency: Developing catalysts that provide high efficiency and selectivity in isotopic labeling reactions is a key area of focus. Current catalysts may not always deliver the desired yield, necessitating the exploration of new catalytic systems.
- Reaction Optimization: Fine-tuning reaction conditions, such as temperature, pressure, and solvent systems, is essential for maximizing yield. This optimization process can be time-consuming and requires a deep understanding of the reaction mechanisms involved.
Future Research Goals
To overcome these challenges, future research in isotopic labeling aims to focus on sustainability and the reduction of radioactive waste. These goals are essential for ensuring the long-term viability and environmental safety of isotopic labeling practices.
Sustainability
Sustainability in isotopic labeling involves developing methods that are not only efficient but also environmentally friendly. This includes:
- Green Chemistry: Incorporating principles of green chemistry to minimize the use of hazardous reagents and reduce the environmental impact of isotopic labeling processes. This approach seeks to make the synthesis of labeled compounds safer and more sustainable.
- Renewable Resources: Exploring the use of renewable resources and alternative energy sources in isotopic labeling processes. This can help reduce the dependency on non-renewable materials and lower the overall environmental footprint.
Reduction of Radioactive Waste
Reducing the amount of radioactive waste generated during isotopic labeling is a critical goal for the future. Strategies to achieve this include:
- Efficient Use of Radioisotopes: Developing methods that maximize the utilization of radioisotopes, ensuring that minimal amounts are wasted. This involves optimizing reaction conditions and improving the efficiency of labeling processes.
- Waste Management: Implementing effective waste management practices to safely dispose of radioactive materials. This includes developing technologies for the safe containment and disposal of radioactive waste, as well as exploring recycling and reuse options where feasible.
By focusing on these future research goals, the field of isotopic labeling can continue to advance, providing valuable tools for pharmaceutical research while minimizing environmental impact and enhancing sustainability.
Conclusion
Isotopic labeling stands out as a pivotal technique in pharmaceutical research, offering unparalleled insights into the behavior of drugs within biological systems. This powerful method allows scientists to trace the pathways of drugs, understand their interactions, and study their pharmacokinetics and pharmacodynamics with high precision. By incorporating isotopes such as deuterium, tritium, carbon-14, and others into molecules, researchers can observe how drugs are absorbed, distributed, metabolized, and excreted, which is crucial for the development of safe and effective pharmaceuticals.
Recap of the Importance of Isotopic Labeling in Pharmaceutical Research
The importance of isotopic labeling in pharmaceutical research cannot be overstated. It provides a detailed understanding of drug mechanisms at the molecular level, which is essential for:
- Drug Development: Enhancing the stability and efficacy of drugs through techniques like deuterium labeling, which improves pharmacokinetic properties.
- Early-Stage Discovery: Utilizing tritium labeling for in vitro studies and binding assays to explore drug-receptor interactions.
- Metabolic Studies: Employing carbon-14 labeling to conduct comprehensive ADME studies and first-in-human trials, ensuring the safety and efficacy of new drug candidates.
- Innovative Techniques: Advancements in hydrogen isotope exchange (HIE) and late-stage functionalization have expanded the scope and efficiency of isotopic labeling, making it more versatile and applicable to a wider range of compounds.
Final Thoughts on Future Advancements and Potential Impacts on Drug Development
Looking forward, the field of isotopic labeling is poised for significant advancements. Future research is expected to focus on:
- Sustainability: Developing greener and more sustainable labeling techniques that minimize environmental impact and radioactive waste.
- Efficiency: Optimizing reaction conditions and catalysts to achieve higher yields and reduce the complexity of synthesizing labeled compounds.
- Collaborative Innovation: Continued collaboration between academia and industry will drive the development of new methodologies and enhance the application of isotopic labeling in various scientific domains.
These advancements will have profound impacts on drug development, enabling the creation of safer, more effective drugs with improved pharmacokinetic profiles. As isotopic labeling techniques become more refined and accessible, they will continue to play a critical role in the discovery and development of next-generation therapeutics, ultimately improving patient outcomes and advancing the field of pharmaceutical science.
Reference
- Sib, A., & Derdau, V. (2024). Method Development and Syntheses Examples of Isotopically Labeled Compounds to Foster Operational Excellence in Pharma Industry. Synlett.
- Mutlib, A. E. (2008). Application of stable isotope-labeled compounds in metabolism and in metabolism-mediated toxicity studies. Chemical research in toxicology, 21(9), 1672-1689.
- Creek, D. J., Chokkathukalam, A., Jankevics, A., Burgess, K. E., Breitling, R., & Barrett, M. P. (2012). Stable isotope-assisted metabolomics for network-wide metabolic pathway elucidation. Analytical chemistry, 84(20), 8442-8447.
- Atzrodt, J., Derdau, V., Kerr, W. J., & Reid, M. (2018). Deuterium‐and tritium‐labelled compounds: applications in the life sciences. Angewandte chemie international edition, 57(7), 1758-1784.




