Unveiling Neuroimmune Communication: The Role of Adrenergic Receptor Signaling in Health and Disease
Abstract
Adrenergic receptor signaling plays a pivotal role in neuroimmune communication, bridging the central nervous system and the immune system to maintain homeostasis and respond to environmental challenges. This intricate interplay regulates immune cell activation, cytokine production, and inflammatory responses through the activation of adrenergic receptors. These receptors exhibit subtype-specific effects across various immune cells, influencing disease progression in conditions such as cancer, autoimmune disorders, and infectious diseases. Recent research highlights the therapeutic potential of modulating adrenergic signaling to restore immune balance and enhance treatment efficacy. Future directions include developing receptor-specific modulators, understanding pathway cross-talk, and leveraging precision medicine to optimize outcomes. Advancing this knowledge could pave the way for innovative interventions in immune-related diseases.
Introduction to Neuroimmune Communication
The sympathetic nervous system (SNS) plays a vital role in maintaining the homeostasis of the body by secreting different neurotransmitters like catecholamines, acetylcholine, glutamine, etc. Among all the neurotransmitters, catecholamine has a very important and diversified role in the various organs. Catecholamines contain a catechol (3,4 dihydroxyphenyl) group along with an amine group. John Jacob Abel, in 1897 first obtained a crystalline substance from the adrenal gland of sheep in an impure form that can regulate blood pressure, and he named it epinephrine. In 1900, Jokichi Takamine obtained a pure crystalline form of epinephrine. British Approved Name (BAN) introduced the name “adrenaline” in the United Kingdom and British Commonwealth. Still, United States Approved Name (USAN) used the term “epinephrine” for this neurotransmitter in the USA. While the British Approved Name (BAN) adopted “adrenaline,” the United States Approved Name (USAN) used “epinephrine,” creating a naming controversy. To address such issues, the Recommended International Nonproprietary Name (rINN) system was developed, yet adrenaline and noradrenaline remain exceptions. Catecholamines, including epinephrine and norepinephrine, serve as key neurotransmitters in neuroimmune communication. These molecules are synthesized by chromaffin cells in the adrenal medulla and some CNS regions, such as the locus coeruleus. Upon release, catecholamines bind to adrenergic receptors expressed on immune and non-immune cells, initiating signaling cascades that influence immune function. For example, activation of β-adrenergic receptors typically leads to immune suppression, characterized by reduced cytokine production and diminished phagocytic activity in macrophages. Conversely, α-adrenergic receptors can exhibit pro-inflammatory or anti-inflammatory effects depending on the cellular context. Both epinephrine and norepinephrine stimulate common receptors named adrenergic receptors. Structurally these receptors have seven hydrophobic transmembrane regions and an intracellular C-terminal domain and an extracellular N-terminal domain, along with 3 intracellular and extracellular loops. The N-terminal domain contains sites for N-linked glycosylation. Based on specificity to epinephrine, adrenergic receptors are broadly divided into three categories, α1, α2, and β adrenergic receptors. The α1 and α2 are again subdivided into α1A, α1B, α1D and α2A, α2B, and α2C, respectively. β adrenergic receptor is divided into β1, β2, and β3. These receptors and their tissue distribution are listed in Table 1. These receptors resemble a serpentine structure, but they vary in the intracellular C terminal region. Understanding neuroimmune communication, particularly through adrenergic receptor signaling, provides a foundation for developing innovative therapeutic strategies. By leveraging this knowledge, researchers aim to design targeted interventions that modulate adrenergic signaling, potentially offering new treatments for conditions such as autoimmune diseases and immunological dysfunctions.Adrenergic Receptors: Types and Functions
Adrenergic receptors are critical components of the neuroimmune communication system, serving as the molecular interface through which catecholamines such as epinephrine and norepinephrine exert their effects. These receptors are classified into three main types: α1, α2, and β, with each type further subdivided into subtypes (e.g., α1A, α1B, α1D; α2A, α2B, α2C; and β1, β2, β3). These subtypes differ in their tissue distribution, ligand specificity, and downstream signaling pathways. Structurally, adrenergic receptors belong to the G protein-coupled receptor (GPCR) family, characterized by seven transmembrane domains. Upon activation, these receptors interact with specific G proteins (Gs, Gi, or Gq), triggering distinct signaling cascades. For instance, β-adrenergic receptors primarily couple with Gs proteins, leading to the activation of adenylate cyclase and subsequent production of cyclic AMP (cAMP). This cAMP acts as a second messenger, modulating various cellular processes such as cytokine production, phagocytosis, and cell migration. In contrast, α1-adrenergic receptors couple with Gq proteins, activating phospholipase C and generating inositol triphosphate (IP3) and diacylglycerol (DAG). These molecules increase intracellular calcium levels, promoting vasoconstriction and other cellular functions. Meanwhile, α2-adrenergic receptors are linked to Gi proteins, which inhibit adenylate cyclase activity, reduce cAMP levels, and modulate processes like neurotransmitter release. The physiological roles of adrenergic receptors extend beyond immune modulation. They regulate cardiovascular function, metabolism, and stress responses. However, their role in immune cells, such as T cells, macrophages, and dendritic cells, is particularly intriguing. β2-adrenergic receptors, for example, are associated with immune suppression, while α1 and α2 receptors can have dual effects depending on the context.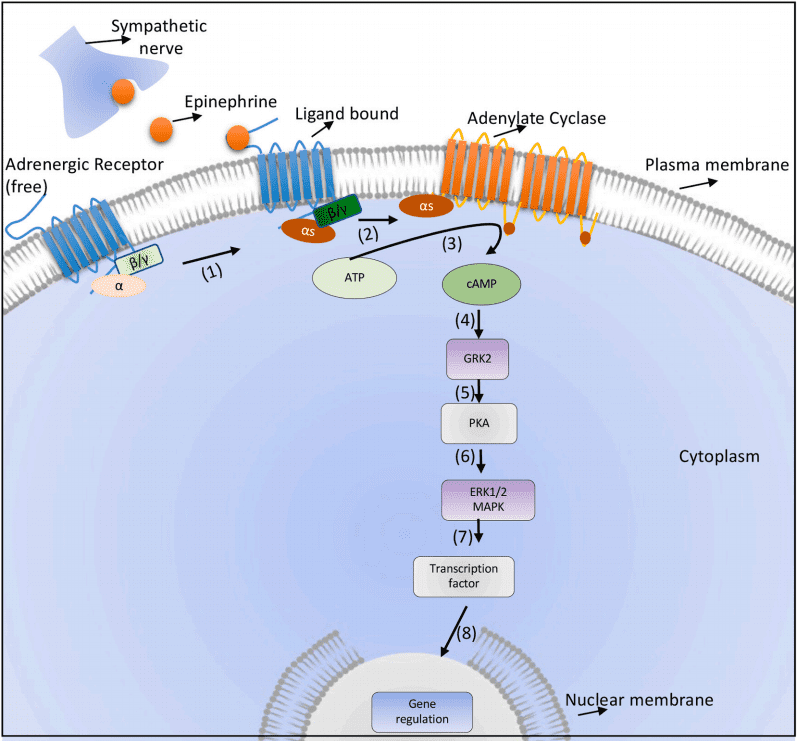
Fig. 1. Canonical β2 adrenergic receptor signalling
The broad expression of adrenergic receptors in immune and non-immune cells makes them attractive therapeutic targets. Understanding the nuances of adrenergic receptor signaling, including subtype-specific effects and cross-talk with other signaling pathways, is crucial for developing targeted interventions for diseases ranging from cancer to autoimmune disorders.Adrenergic Receptors in Immune Cells
Adrenergic receptors, widely expressed on immune cells, are key mediators of neuroimmune interactions. These receptors play a pivotal role in regulating immune cell function, enabling the body to adapt to stress, inflammation, and infections. Both innate and adaptive immune cells express various adrenergic receptor subtypes, including α1, α2, and β-adrenergic receptors, which influence their activation, differentiation, and effector responses.Dendritic Cells (DCs)
Dendritic cells are antigen-presenting cells crucial for initiating and regulating immune responses. These cells express α1, α2, and β2-adrenergic receptors, which modulate their activity. For instance, norepinephrine binding to β2-adrenergic receptors suppresses pro-inflammatory cytokine production, such as interleukin-12 (IL-12), while enhancing anti-inflammatory cytokines like IL-10. This signaling shift influences the balance of T-helper cell polarization, promoting Th2 responses over Th1.Monocytes and Macrophages
Monocytes and macrophages are essential for innate immunity and tissue repair. Adrenergic signaling in these cells primarily occurs through β2-adrenergic receptors, which exhibit immunosuppressive effects. Activation of β2 receptors reduces tumor necrosis factor-alpha (TNF-α) production and impairs phagocytosis. However, in some contexts, α1-adrenergic receptor signaling in macrophages promotes pro-inflammatory activities. These dual roles highlight the context-dependent nature of adrenergic receptor effects.T and B Cells
T cells and B cells express α1, α2, and β2-adrenergic receptors, which regulate their activation and function. In T cells, β2-adrenergic receptor activation suppresses IL-2 production, thereby reducing their proliferation and effector functions. Similarly, B cells exhibit reduced antibody production upon β2 receptor activation. These mechanisms underscore the suppressive role of adrenergic signaling in adaptive immunity, crucial for maintaining immune homeostasis.Natural Killer (NK) Cells
Natural killer cells, which play a vital role in antiviral and anticancer immunity, are also regulated by adrenergic signaling. Activation of β2-adrenergic receptors inhibits NK cell cytotoxicity and cytokine secretion, potentially contributing to immune evasion in stressful conditions. Adrenergic signaling in immune cells thus represents a complex, context-specific system with implications for disease progression and therapeutic interventions.Clinical Implications and Disease Relevance
The role of adrenergic receptor signaling extends beyond physiological regulation, significantly influencing the progression of various diseases. This signaling pathway has been implicated in cancer, autoimmune disorders, and infectious diseases, positioning adrenergic receptors as potential therapeutic targets.Cancer
In cancer, adrenergic signaling has been shown to promote tumor growth and metastasis. β2-adrenergic receptor activation enhances the secretion of pro-tumorigenic cytokines like vascular endothelial growth factor (VEGF) and interleukin-6 (IL-6), facilitating angiogenesis and tumor progression. Stress-induced activation of adrenergic signaling can suppress immune responses by inhibiting the activity of natural killer (NK) cells and cytotoxic T lymphocytes, leading to an immune-evasive tumor microenvironment. Therapeutically, β-blockers such as propranolol have demonstrated efficacy in reducing tumor growth and improving outcomes in cancers like melanoma and breast cancer.Autoimmune Disorders
Adrenergic receptor signaling plays a dual role in autoimmune diseases, with context-dependent effects on immune modulation. In conditions like rheumatoid arthritis (RA), β2-adrenergic receptor activation reduces inflammatory cytokine production and limits immune cell proliferation, suggesting a protective role. Conversely, chronic stress or dysregulated adrenergic signaling can exacerbate autoimmune responses by altering T-cell differentiation and promoting a Th1/Th17-dominated inflammatory environment. These findings highlight the therapeutic potential of adrenergic receptor modulation in autoimmune conditions.Infectious Diseases
Adrenergic receptors also influence the immune response to infections. During bacterial and viral infections, β2-adrenergic receptor activation suppresses pro-inflammatory cytokine production, potentially impairing pathogen clearance. However, in certain contexts, adrenergic signaling can regulate excessive inflammation, preventing tissue damage and cytokine storms. For instance, in severe COVID-19 cases, α-adrenergic receptor antagonists have shown promise in mitigating hyperinflammatory responses and reducing mortality rates. The clinical relevance of adrenergic receptor signaling lies in its ability to modulate immune responses in a context-specific manner. By targeting specific adrenergic receptor subtypes, it may be possible to develop tailored therapies for a wide range of diseases, from cancer to autoimmune and infectious diseases.Future Directions in Neuroimmune Research
The study of adrenergic receptor signaling in neuroimmune communication has opened new avenues for understanding and treating immune-related diseases. However, several knowledge gaps remain that must be addressed to unlock the full therapeutic potential of this system. A critical area for future research involves the context-specific effects of adrenergic signaling on different immune cells. While β2-adrenergic receptors predominantly exhibit immunosuppressive functions, their effects can vary significantly depending on the type of immune cell and disease state. For example, adrenergic signaling suppresses macrophage phagocytosis but can promote anti-inflammatory M2 polarization under certain conditions. Understanding these nuanced roles will help design targeted interventions. Another promising direction is the development of selective adrenergic receptor modulators. Most current treatments, such as β-blockers, lack receptor subtype specificity, potentially leading to off-target effects. Advances in molecular pharmacology could enable the creation of drugs that precisely target specific adrenergic receptor subtypes, enhancing therapeutic efficacy while minimizing adverse effects. For instance, selective β2-adrenergic receptor antagonists could be optimized for cancer immunotherapy to enhance natural killer (NK) cell activity and T-cell-mediated tumor destruction. The integration of adrenergic signaling with other immune-regulatory pathways also warrants further investigation. Cross-talk between adrenergic signaling and pathways such as NF-κB, JAK-STAT, and MAPK may reveal novel therapeutic targets. Understanding how adrenergic signaling interacts with these pathways in various disease contexts, such as autoimmunity, cancer, and infections, can inform combination therapies. Recently, neuroimmune communication in modulating the adaptive and innate immune response is getting much attention in different inflammatory and autoimmune diseases and cancer. The sympathetic nervous system (SNS) controls T cell responses induced during viral and parasitic infection as well as in anti-tumour immunity . Most of the immune cells are known to express adrenergic receptors, and the function of these receptors plays an important role in inflammation. In most cases, activation of β2 adrenergic receptor shows an immune-suppressive function which may vary in different disease conditions. The sympathetic nervous system controls both the immune system’s innate and adaptive branches via these adrenergic receptors. Pharmacological interventions showed that adrenergic receptors play a significant role in modulating the immune response. The direction of the adrenergic system in specific cell types, in specific tissues and how it controls the cross talk among the various immune cells in shaping the immune response needs a systematic and well-defined experimental strategy. The adrenergic signalling is mostly studied in the vasodilation and blood flow in the tissues. Recently, it was found that noradrenaline administration promoted the constriction of post-capillary venules and artrioles in the lymph nodes, which had impact on immune cell interaction in the secondary lymphoid organs and immune response. As adrenergic signalling has an immense role in different diseases, specific pharmacological agonists and antagonists targeting a defined adrenergic receptor in specific diseases could be explored for better clinical outcomes. A better understanding of the cellular and molecular mechanisms of adrenergic receptors in various immune cells and their importance will help explore the therapeutic repurposing of known agonists and antagonists.References
- Chhatar, S., & Lal, G. (2021). Role of adrenergic receptor signalling in neuroimmune communication. Current Research in Immunology, 2, 202–217. DOI
- Scanzano, A., & Cosentino, M. (2015). Adrenergic regulation of innate immunity: A review. Frontiers in Pharmacology, 6, 171. DOI
- Cosentino, M., & Marino, F. (2013). Adrenergic and dopaminergic modulation of immunity in rheumatoid arthritis. Pharmacology Research & Perspectives, 1(1), e00006. DOI
- Vasanthakumar, N. (2020). Can beta-adrenergic blockers be used in the treatment of COVID-19? Medical Hypotheses, 142, 109809. DOI
- Baker, A. J., & Fuller, R. W. (1995). Loss of response to beta-adrenoceptor agonists during the maturation of human monocytes to macrophages in vitro. Journal of Leukocyte Biology, 57, 395–400. Link