Unveiling the Dual Role of NDRG1 in Breast Cancer: Pathways to Personalized Treatment
Abstract
Breast cancer remains one of the most prevalent and challenging diseases, affecting millions of women worldwide. This blog post delves into the complexities of breast cancer, emphasizing the significance of molecular subtyping in guiding treatment and improving prognoses. It highlights the pivotal role of N-myc downstream-regulated gene-1 (NDRG1), a protein that functions both as a metastasis suppressor and a pro-oncogenic factor, underscoring the dual nature of its impact on cancer pathogenesis and therapy. Innovative approaches in targeting NDRG1 through emerging therapies are discussed, offering new hope for treating aggressive and resistant forms of breast cancer, such as triple-negative breast cancer (TNBC). The post also explores the importance of personalized medicine and the future of cancer treatment, focusing on the integration of molecular biology into clinical practice to tailor therapies that are more effective and less burdensome for patients. By understanding and leveraging the molecular drivers of breast cancer, significant strides can be made towards more effective treatments and ultimately, better patient outcomes.
Understanding Breast Cancer: An Introduction to Its Complexity and Molecular Diversity
Breast cancer remains the most prevalent and deadly cancer affecting women globally, accounting for a significant proportion of cancer-related deaths each year. This disease is not a single entity; rather, it manifests in multiple forms, each with its distinct pathological and molecular characteristics. The complexity of breast cancer stems from its classification into various subtypes, which are primarily determined based on the presence or absence of certain receptors, such as the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2).
Molecular subtyping of breast cancer is crucial as it directly influences treatment decisions and prognostic evaluations. Typically, breast cancers are categorized into three main subtypes: ER-positive, HER2-positive, and triple-negative breast cancer (TNBC). ER-positive cancers, which express the estrogen receptor, are often treated with hormone-blocking therapies like tamoxifen or aromatase inhibitors. HER2-positive cancers, which show overexpression of the HER2 protein, can be targeted with drugs such as trastuzumab and pertuzumab, designed to inhibit HER2-driven cell proliferation. 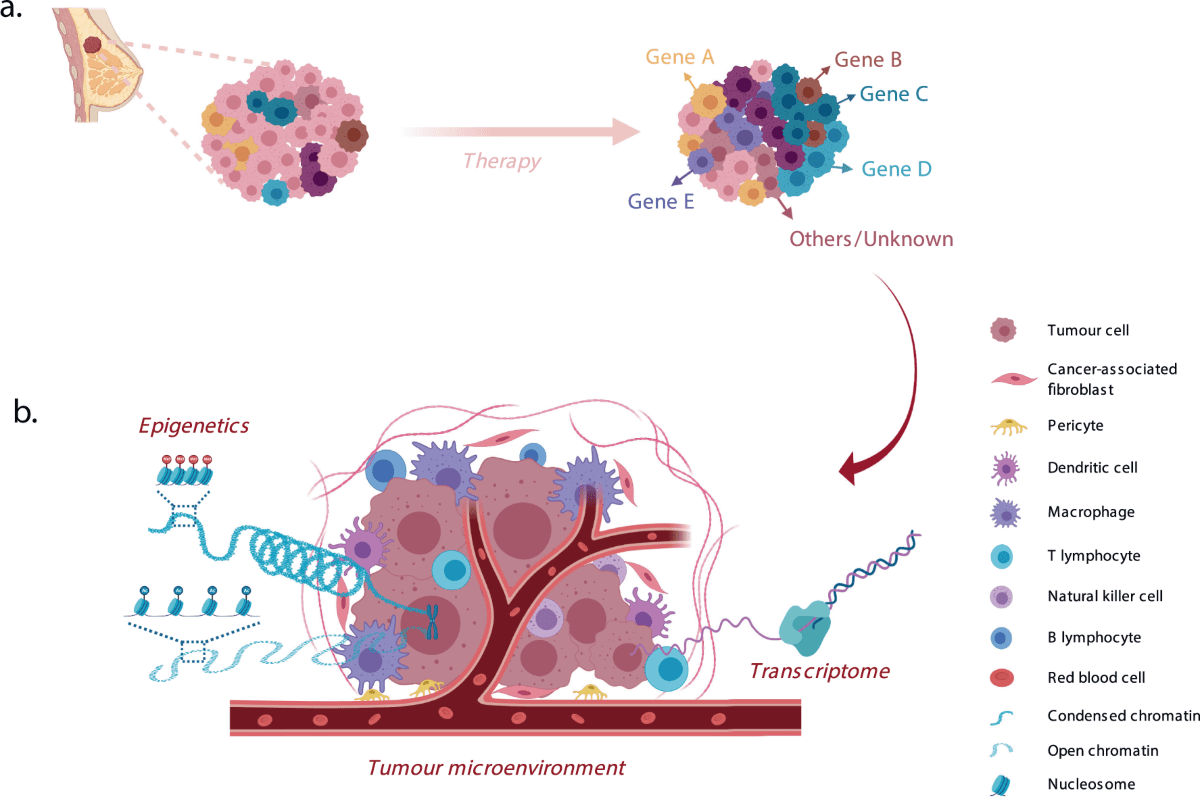
Figure 1 Pre-existing and acquired mechanisms of resistance to therapy in a model of clonal selection.
Conversely, TNBC lacks the expression of ER, PR, and HER2 and poses significant treatment challenges due to the absence of well-defined targets. The heterogeneity within these subtypes further complicates treatment approaches, as patients with seemingly similar diagnoses might respond differently to the same therapy. Understanding this molecular diversity not only helps in customizing treatment plans but also underscores the need for ongoing research to discover more about the underlying mechanisms of each subtype, leading to more effective and personalized therapies.
Spotlight on NDRG1: A Key Player in Breast Cancer Dynamics
One of the intriguing aspects of breast cancer research is the discovery of genes that play critical roles in the disease’s pathogenesis and treatment responses. N-myc downstream-regulated gene-1 (NDRG1) is a protein that has garnered significant attention due to its dual roles in cancer biology. NDRG1 can function both as a metastasis suppressor and, paradoxically, as a pro-oncogenic factor, depending on the cellular context and cancer type.

Figure 2 Schematic representation of the protein structure of NDRG1
NDRG1’s role as a metastasis suppressor is well-documented in various cancers, including breast cancer. It helps in inhibiting the process known as epithelial-to-mesenchymal transition (EMT), a critical mechanism through which cancer cells acquire the ability to invade, migrate, and ultimately form metastases. By regulating this process, NDRG1 can potentially prevent the spread of cancer cells from the primary tumor to distant organs.
However, the complexity of NDRG1’s function extends beyond its suppressive activities. In breast cancer, NDRG1 has also been implicated in promoting oncogenic signaling pathways related to lipid metabolism and the mTOR signaling pathway. This dual functionality makes NDRG1 a fascinating subject for researchers aiming to understand how its expression and regulatory mechanisms can be manipulated to develop more effective cancer therapies.
Given the significant impact of NDRG1 on breast cancer outcomes, it is crucial to explore therapeutic strategies that could modulate its activity. Innovations in drug development that target NDRG1 could offer new hope for patients, particularly those with aggressive subtypes like triple-negative breast cancer, where current treatment options are limited.
NDRG1’s Pivotal Influence on Breast Cancer Treatment Outcomes
The potential of NDRG1 to impact breast cancer treatment outcomes is substantial, making it a critical focal point for developing personalized cancer therapies. NDRG1’s expression levels are not just a biological marker but may determine the success or failure of specific treatments. For instance, high levels of NDRG1 have been associated with improved responsiveness to certain chemotherapy agents, suggesting its role as a biomarker for treatment efficacy.
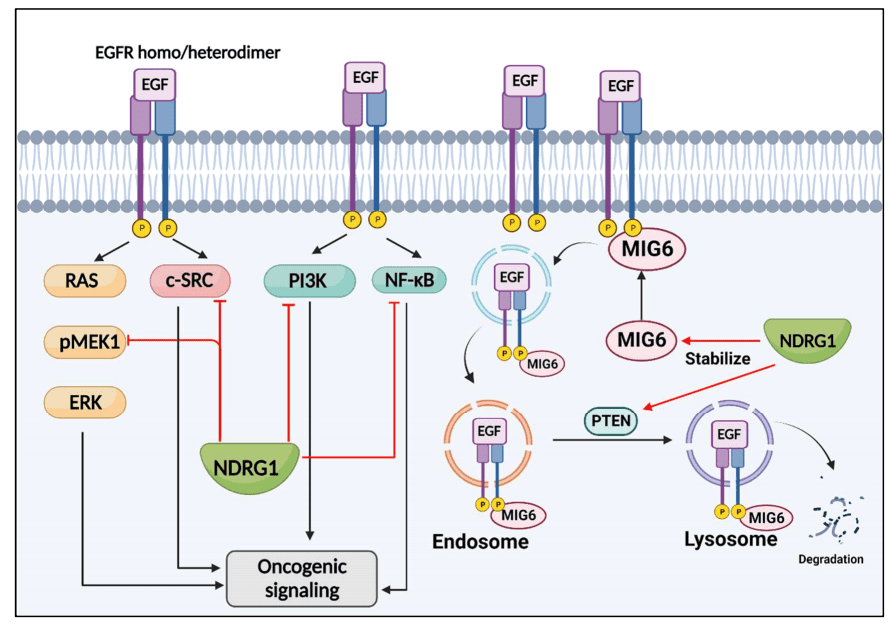
Figure 3 NDRG1 suppresses EGFR signaling.
Emerging research indicates that therapies aimed at modulating the expression of NDRG1 could provide breakthroughs in treating resistant forms of breast cancer, such as triple-negative breast cancer (TNBC). TNBC lacks targeted therapies due to the absence of estrogen, progesterone, and HER2 receptors, making it notoriously difficult to treat. Pharmacological agents that can increase NDRG1 expression may inhibit cancer cell migration and metastasis, offering a novel approach to managing this aggressive cancer subtype.
Moreover, the development of new pharmacological strategies that target NDRG1 directly is on the horizon. These include small molecule inhibitors and antibody-based therapies that either inhibit its oncogenic functions or enhance its tumor suppressor activities. For example, di-2-pyridylketone thiosemicarbazone derivatives have shown promise in preclinical studies for up-regulating NDRG1 and inhibiting cancer cell growth and metastasis.
As research progresses, the therapeutic landscape for breast cancer is poised to be significantly impacted by how we leverage our understanding of NDRG1’s dual roles. This could lead to more effective, tailored therapies that improve survival rates and quality of life for patients battling this complex disease.
Innovative Approaches to Breast Cancer Treatment: The Future is Now
As the fight against breast cancer continues, the scientific community is increasingly looking toward innovative approaches that target specific molecular pathways to improve treatment efficacy and patient outcomes. One such promising pathway involves the protein NDRG1, whose complex role in cancer biology presents unique opportunities for therapeutic intervention.
Recent advancements in understanding the molecular drivers of breast cancer have paved the way for novel treatments that are more precise and effective. For instance, targeted therapy using drugs that specifically enhance or inhibit NDRG1’s activity is emerging as a promising strategy, particularly for aggressive and treatment-resistant breast cancer subtypes such as triple-negative breast cancer (TNBC). These targeted therapies could potentially revolutionize the approach to cancer treatment by directly influencing the molecular pathways that drive tumor growth and metastasis.
Clinical trials are crucial in this context as they help determine the safety and efficacy of new drugs targeting NDRG1. Ongoing and upcoming clinical trials focusing on NDRG1-related therapies are being closely watched by the medical community for their potential to set new standards in cancer care. For example, trials using NDRG1 modulators to test their ability to suppress tumor metastasis or reverse resistance to existing therapies are in various phases, providing hope for breakthroughs in the near future.
The future of breast cancer treatment is also seeing a shift towards more personalized approaches. By understanding a patient’s specific genetic and molecular profile, treatments can be tailored to target the unique aspects of their tumor, thereby increasing the effectiveness of the therapy and reducing potential side effects.
Conclusion: Empowering Through Knowledge in the Fight Against Breast Cancer
The journey through understanding and combating breast cancer continues to evolve, with precision medicine and targeted therapies at the forefront of promising treatment avenues. The protein NDRG1 exemplifies the intricate balance of promoting and inhibiting cancer, showcasing the complex nature of the disease and highlighting the nuanced approaches needed for effective treatment. This complexity underscores the importance of molecular research in unveiling new therapeutic potentials that can significantly improve patient outcomes, particularly for aggressive forms such as triple-negative breast cancer.
As we navigate this challenging landscape, staying informed about the progress in breast cancer research becomes crucial. Advances in molecular biology and treatment strategies offer hope and new opportunities for managing the disease more effectively. By engaging with the latest research findings and understanding the significant role of proteins like NDRG1, patients, healthcare providers, and the wider community can better navigate the options available for treatment and care.
The progress in breast cancer treatment not only represents a beacon of hope for those affected but also serves as a testament to the relentless pursuit of knowledge by the scientific community. It is through continued research and enhanced understanding that we can look forward to more breakthroughs that will eventually lead to better management, and hopefully, cures for breast cancer.




