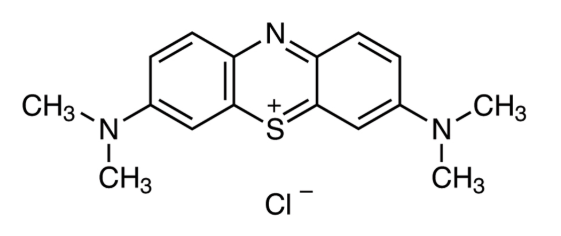
Vinorelbine–Thiotepa Combination Shows Promise in Metastatic Breast Cancer with CNS Involvement: Real-World Insights from a Retrospective Study
Abstract
Metastatic breast cancer (MBC), particularly with central nervous system (CNS) involvement, presents major therapeutic challenges due to limited treatment options that effectively cross the blood–brain barrier. A recent real-world retrospective study evaluated the combination of vinorelbine and thiotepa in 137 patients with advanced MBC, including a substantial subgroup with brain and leptomeningeal metastases. The study reported a median progression-free survival of 4.4 months and an overall survival of 12.7 months, with notable activity in heavily pretreated and triple-negative subtypes. Although the regimen was associated with hematologic toxicity, it was generally manageable. These findings highlight the potential role of vinorelbine–thiotepa as a salvage therapy for MBC patients, especially those with CNS disease, and support the need for prospective clinical trials.
Revisiting Treatment Options in Metastatic Breast Cancer
Metastatic breast cancer (MBC) remains one of the most complex and challenging stages of breast cancer to treat. Despite advancements in targeted therapies and immunotherapy, the disease is still considered incurable, and treatment often focuses on prolonging survival while maintaining quality of life. One of the major hurdles in managing MBC is the heterogeneity of the disease and the frequent emergence of resistance to standard therapies like taxanes and anthracyclines.
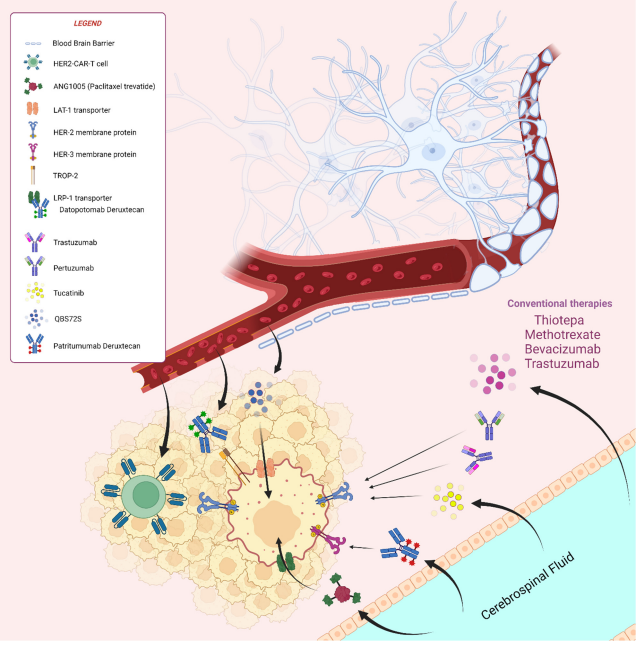
Figure 1. Overview of old and new treatment strategies in the management of brain metastases from breast cancer
An additional layer of complexity arises when cancer spreads to the central nervous system (CNS). CNS metastases, including brain and leptomeningeal involvement, are increasingly common due to improved survival in MBC patients. Unfortunately, many chemotherapy agents have limited efficacy in the brain because they cannot effectively cross the blood–brain barrier (BBB). This limits treatment options for a growing subset of patients with CNS metastases.
While novel agents like CDK4/6 inhibitors and antibody-drug conjugates have reshaped first- and second-line therapy landscapes, cytotoxic chemotherapy remains a critical component of salvage treatment strategies. In this context, vinorelbine, a semi-synthetic vinca alkaloid, has established itself as a commonly used chemotherapy drug for MBC. When used alone or in combination, it has shown meaningful response rates, particularly in patients pretreated with anthracyclines or taxanes.
Thiotepa, a less commonly used chemotherapeutic agent, offers a unique advantage: its lipophilic structure allows it to penetrate the BBB, making it a potentially valuable option for patients with CNS involvement. Despite its theoretical benefits, the vinorelbine–thiotepa combination has not been extensively studied in real-world settings—until recently.
As clinicians seek more effective strategies for advanced and hard-to-treat cases, the combination of vinorelbine and thiotepa emerges as a compelling option worth deeper investigation.
Study Spotlight: Evaluating Vinorelbine and Thiotepa in the Real World
While clinical trials offer crucial insights into drug efficacy, real-world data plays an equally important role in understanding how treatments perform outside the controlled environment of research settings. A recent large retrospective cohort study, conducted at Saint Louis Hospital in Paris, has brought new attention to an underexplored combination therapy for metastatic breast cancer (MBC): vinorelbine and thiotepa.
This observational study analyzed data from 137 patients with histologically confirmed MBC who were treated with intravenous vinorelbine and thiotepa over a 10-year period. All patients had previously received standard chemotherapies like anthracyclines and/or taxanes, and a significant proportion had already undergone multiple lines of systemic treatment in the metastatic setting. Importantly, more than one-third of the study population had central nervous system (CNS) involvement, including brain metastases and leptomeningeal carcinomatosis—areas notoriously difficult to treat due to the protective nature of the blood–brain barrier.
Vinorelbine, commonly used in MBC management, has demonstrated efficacy in both first- and later-line treatment settings. Thiotepa, while less frequently used, is known for its ability to cross the blood–brain barrier, making it particularly intriguing for patients with CNS metastases. However, the combination of these two agents has not been widely adopted, and until now, evidence for its real-world effectiveness and safety profile has been sparse.
By capturing the experiences of unselected, heavily pretreated patients in routine clinical practice, this study offers valuable insights into how vinorelbine–thiotepa may benefit those with advanced or aggressive disease. It sets the stage for deeper evaluation of this regimen, especially for MBC cases with limited therapeutic options, such as triple-negative subtypes and CNS involvement.
Key Findings: Efficacy and Survival Outcomes
The real-world study of vinorelbine and thiotepa in metastatic breast cancer (MBC) delivers meaningful insights into the efficacy of this combination, especially in patients with aggressive disease or central nervous system (CNS) involvement. While both drugs have individually shown therapeutic value in breast cancer management, their combined use has been understudied—until now.
In this retrospective analysis of 137 patients, the median progression-free survival (PFS) was 4.4 months in the overall population. Notably, patients with CNS metastases—who typically face worse outcomes due to the limitations imposed by the blood–brain barrier—achieved a median PFS of 3.6 months. Median overall survival (OS) was reported at 12.7 months for the general cohort and 10.3 months for those with CNS involvement. These results are significant, given that many patients had already undergone multiple lines of therapy and presented with advanced disease features such as triple-negative breast cancer (TNBC) and widespread metastasis.
For highly pretreated patients (those who had received three or more prior therapies at the metastatic stage), the median PFS was 3.5 months. Interestingly, in patients with metastatic TNBC—a particularly aggressive and hard-to-treat subtype—the median PFS was 4.5 months, comparable to other approved therapies like capecitabine.
These findings underscore the potential of vinorelbine–thiotepa as a viable salvage treatment, particularly for MBC patients with limited options. While the efficacy results may not dramatically exceed those of existing regimens, the combination’s activity in CNS-involved cases offers a promising avenue for further clinical investigation. Given thiotepa’s ability to cross the blood–brain barrier and vinorelbine’s established role in breast cancer treatment, this duo could address an important unmet need in the oncology landscape.
Safety and Limitations: Balancing Benefits and Risks
While the vinorelbine–thiotepa combination demonstrated clinical activity in metastatic breast cancer (MBC), especially among patients with central nervous system (CNS) involvement, its use is not without challenges—particularly regarding safety and study design limitations.
In the study cohort of 137 patients, 35% experienced Grade 3 or 4 adverse events. The most frequent high-grade toxicity was hematologic, including neutropenia and anemia, leading to dose reductions in more than one-third (36.5%) of participants. Other significant side effects included perineural neuropathy and digestive toxicity, although these were less common. Only 5.1% of patients had to discontinue treatment due to toxicity, and importantly, no treatment-related deaths were reported.
Despite these manageable side effects, the toxicity profile should not be overlooked, especially in heavily pretreated and often frail patients with advanced MBC. Careful patient selection and close monitoring are essential when considering this regimen in routine clinical practice.
From a methodological perspective, the study’s retrospective and single-center design introduces potential biases. Not all patients underwent standardized radiological assessments, making it difficult to uniformly evaluate treatment response. In addition, the inclusion of patients who received concurrent CNS-targeted therapies (such as radiotherapy or intrathecal chemotherapy) adds further complexity when interpreting the results—particularly for the subgroup with CNS metastases.
Moreover, the patient population was highly heterogeneous, both in terms of prior treatments and tumor biology. For example, only a small proportion of hormone receptor-positive patients had received CDK4/6 inhibitors, which are now standard in the metastatic setting. This raises questions about how applicable the results are to today’s evolving treatment landscape.
Conclusion: Is Vinorelbine–Thiotepa a Viable Option for MBC with CNS Spread?
The retrospective study on vinorelbine and thiotepa provides important real-world evidence supporting the use of this combination in metastatic breast cancer (MBC), particularly in cases with central nervous system (CNS) involvement. In a setting where treatment options are often limited and outcomes are poor, especially for patients with triple-negative subtypes or brain metastases, this regimen offers a ray of hope.
Although progression-free and overall survival outcomes were modest, they are notable considering the complexity and advanced stage of the disease in this patient population. The ability of thiotepa to cross the blood–brain barrier gives this regimen a distinctive advantage over many other chemotherapeutic agents that are ineffective against CNS metastases. When paired with vinorelbine—a well-established treatment in breast cancer—the combination has the potential to fill an important therapeutic gap.
However, the safety profile requires careful consideration. Hematologic toxicity was significant, and dose modifications were frequently necessary. Nonetheless, the absence of treatment-related mortality and the manageable nature of most side effects suggest that the regimen could be safely administered with appropriate monitoring.
Despite the study’s limitations, including its retrospective design and heterogeneity among patients, it sets the stage for future prospective trials. Randomized studies comparing vinorelbine–thiotepa to standard-of-care treatments or placebo could help clarify its role and better define which patients would benefit most.
In conclusion, vinorelbine–thiotepa is a promising option for a subset of MBC patients—particularly those with CNS disease or limited alternatives. As new systemic therapies continue to evolve, this combination deserves further exploration as part of the expanding toolkit for advanced breast cancer management.
References
Finn, R. S., Martin, M., Rugo, H. S., et al. (2016). Palbociclib and letrozole in advanced breast cancer. New England Journal of Medicine, 375(20), 1925–1936. https://doi.org/10.1056/NEJMoa1607303
Kuksis, M., Gao, Y., Tran, W., et al. (2021). The incidence of brain metastases among patients with metastatic breast cancer: A systematic review and meta-analysis. Neuro-Oncology, 23(6), 894–904. https://doi.org/10.1093/neuonc/noaa285
Gregory, R. K., & Smith, I. E. (2000). Vinorelbine—a clinical review. British Journal of Cancer, 82(12), 1907–1913. https://doi.org/10.1054/bjoc.2000.1203
Bonneterre, J., & Penel, N. (2008). Vinorelbine in breast cancer. Expert Opinion on Pharmacotherapy, 9(16), 2901–2910. https://doi.org/10.1517/14656566.9.16.2901
Fabi, A., Tonachella, R., Savarese, A., et al. (1995). A phase II trial of vinorelbine and thiotepa in metastatic breast cancer. Annals of Oncology, 6(2), 187–189. https://doi.org/10.1093/oxfordjournals.annonc.a059115
Loison, R., Abbar, B., Drouin, L., et al. (2023). Vinorelbine thiotepa in metastatic breast cancer: A large real-life retrospective study. Acta Oncologica, 62(12), 1961–1966. https://doi.org/10.1080/0284186X.2023.2260943
Steeg, P. S. (2021). The blood–tumour barrier in cancer biology and therapy. Nature Reviews Clinical Oncology, 18(11), 696–714. https://doi.org/10.1038/s41571-021-00529-6
Chan, A., & Verrill, M. (2009). Capecitabine and vinorelbine in metastatic breast cancer. European Journal of Cancer, 45(13), 2253–2265. https://doi.org/10.1016/j.ejca.2009.04.031
Xu, Y.-C., Wang, H.-X., Tang, L., et al. (2013). A systematic review of vinorelbine for the treatment of breast cancer. The Breast Journal, 19(2), 180–188. https://doi.org/10.1111/tbj.12071
Fabi, A., Tonachella, R., Savarese, A., et al. (1995). A phase II trial of vinorelbine and thiotepa in metastatic breast cancer. Annals of Oncology, 6(2), 187–189. https://doi.org/10.1093/oxfordjournals.annonc.a059115
Steeg, P. S. (2021). The blood–tumour barrier in cancer biology and therapy. Nature Reviews Clinical Oncology, 18(11), 696–714. https://doi.org/10.1038/s41571-021-00529-6