Understanding Isotopically Labeled Compounds: A Comprehensive Guide
Abstract
Isotope labeling is the process of substituting an isotope for the corresponding element in the molecule of a compound. Isotopes are the same element with different masses but the same chemical properties. The method of replacing an atom in a molecule with its isotope is called isotopic labeling. There are two kinds of isotopes, radioactive and non-radioactive. Currently, the radioisotope is commonly used to label in biology. It has the characteristics of convenient detection and high sensitivity. Isotope labeling can be used to study material metabolism, for example, 13N can be used instead of 14N to study the synthesis or breakdown of an N-containing compound in the body. Another example is the use of radioactive 14C instead of 12C to study the metabolism of a certain C atom of a compound. Radioisotope labeling is also used in the detection of antigens, antibodies, and nucleic acids, and can also be used in medical experiments as a means to track the site of drug action and metabolism. A method of replacing common atoms of the same element in a compound molecule with atoms of a radioisotope or stable isotope as tracer atoms to make the entire compound uniquely labeled. On the other hand, labeled compounds differ from ordinary compounds in the ratio of isotopic composition. So isotope labeling is a way to artificially change the isotopic composition of a compound. Isotope labeling methods include the isotope exchange method, biosynthesis method, chemical synthesis method, nuclear reaction method, etc.
What are Isotopically Labeled Compounds?
Isotope is one of two or more atoms of the same chemical element having the same atomic number, occupying the same place on the periodic table, and having nearly identical chemical behavior but differing atomic masses or mass numbers, resulting in differences in mass spectral behavior, radioactive transition, and physical properties (such as diffusion power in the gaseous state). Isotopically labeled compounds refer to the products obtained when one or more atoms or chemical groups in a compound are replaced by their recognizable isotopes or other recognizable nuclides or groups. This process of substitution is called labeling.
What are the common classifications of isotopically labeled compounds?
Isotope labeling is the process of substituting an isotope for the corresponding element in the molecule of a compound. Isotopes are the same element with different masses but the same chemical properties. The method of replacing an atom in a molecule with its isotope is called isotopic labeling. There are two kinds of isotopes, radioactive and non-radioactive. Currently, the radioisotope is commonly used to label in biology. It has the characteristics of convenient detection and high sensitivity. Isotope labeling can be used to study material metabolism, for example, 13N can be used instead of 14N to study the synthesis or breakdown of an N-containing compound in the body. Another example is the use of radioactive 14C instead of 12C to study the metabolism of a certain C atom of a compound. Radioisotope labeling is also used in the detection of antigens, antibodies, and nucleic acids, and can also be used in medical experiments as a means to track the site of drug action and metabolism. A method of replacing common atoms of the same element in a compound molecule with atoms of a radioisotope or stable isotope as tracer atoms to make the entire compound uniquely labeled. On the other hand, labeled compounds differ from ordinary compounds in the ratio of isotopic composition. So isotope labeling is a way to artificially change the isotopic composition of a compound. Isotope labeling methods include the isotope exchange method, biosynthesis method, chemical synthesis method, nuclear reaction method, etc.
The isotope of C is used to label and trace compounds. Using 14C as a tracer, it is widely used in agriculture, chemistry, medicine, and biology. 14C marker compounds can be used to study the photosynthesis of crops, residues of carbon-containing pesticides in soil and crops, etc. It can be used to identify intermediate products of chemical reactions, study reaction kinetics, and reaction pathway, study the formation process of the chemical bond, determine the breaking position of the chemical bond, and study the cause of catalyst poisoning. Used to diagnose diseases (e.g., 14C-xanthine can be used to check liver function) and make low-energy beta sources; Observe the metabolism process of labeled proteins, fats, and amino acids in vivo; To observe the metabolic behavior and elimination of labeled drugs in vivo.
Confirm the Date with 14C. Assuming that the composition of cosmic rays has not changed much over the last 100,000 years, the amount of 14C in the exchanged carbon is a constant. The absolute age of a specimen can be calculated by measuring the specific activity S0 of 14C in the modern carbon that is in the exchange state and the specific activity SA of 14C in the old carbon that is not exchanged. This method can be used in archaeological research to estimate the age of paleontological samples such as wood, bones, hair, and fibrous products from hundreds to tens of thousands of years ago. It can also be widely used in the dating research of geology, geography, oceanography, and meteorology.
The Isotope of Hydrogen: Hydrogen-2 (2H) & Hydrogen-3 (3H)
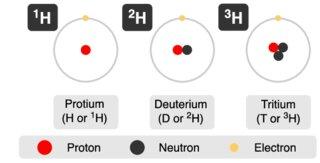
Deuterium is an isotope of hydrogen, also known as heavy hydrogen, with the chemical symbol D or 2H. Deuterium is consisting of a proton, a neutron, and an electron. Deuterium gas is a colorless and odorless flammable gas at room temperature, with an abundance of 0.015% on Earth. Its content in common hydrogen is very small, and most of it exists in the form of heavy water D2O, namely deuterium oxide, in seawater and ordinary water. Tritium, also known as superheavy hydrogen, is one of the isotopes of hydrogen, the element symbol T or 3H. Its nucleus consists of a proton and two neutrons and is radioactive.
Deuterium is used in nuclear energy, controlled nuclear fusion reactions, deuterated optical fiber, deuterated lubricating oil, laser, light bulb, experimental research, semiconductor material toughening treatment, nuclear medicine, nuclear agriculture, and so on. It also has some important military applications, such as hydrogen bombs, neutron bombs, and DF laser weapons. There are also many different methods of making deuterium, depending on its use. With the development and progress of science and technology, deuterium will be used more and more widely, and the demand and research for deuterium will become more important.
Tritium and its labeled compounds play an important role in the military, industry, hydrology, geology, and various scientific research fields. Tritium-labeled compounds are essential tools in many kinds of research in life science. For example, the mechanism and analysis of enzymes, cytology, molecular biology, receptor binding studies, radioimmunoassay, pharmacokinetics, and cancer diagnosis and treatment are all dependent on tritium-labeled compounds. To obtain correct results, attention must be paid to the hydrogen isotope effect, autoradiolysis, and the stability of tritium-labeled compounds under experimental conditions when using tritium-labeled compounds in tracer experiments.
The Isotope of F: Fluoro-18 (18F)
18F is an isotope of fluorine. Fluoro-18 has nine protons and nine neutrons in its nucleus. Its nucleus is unstable, and it is easy to release positrons into oxygen-18, so it is radioactive.
In medicine, Fluoro-18 is mainly used in Positron Emission Tomography (PET) technology, which introduces Fluoro-18 into glucose or drug molecules, decays and produces positrons in the human body after reaching the target, and then emits gamma rays, gamma photons can penetrate human skin tissue. It can be detected by a detector and used as a diagnostic method for tumors.
The Isotope of Oxygen: Oxygen-18 (18O)
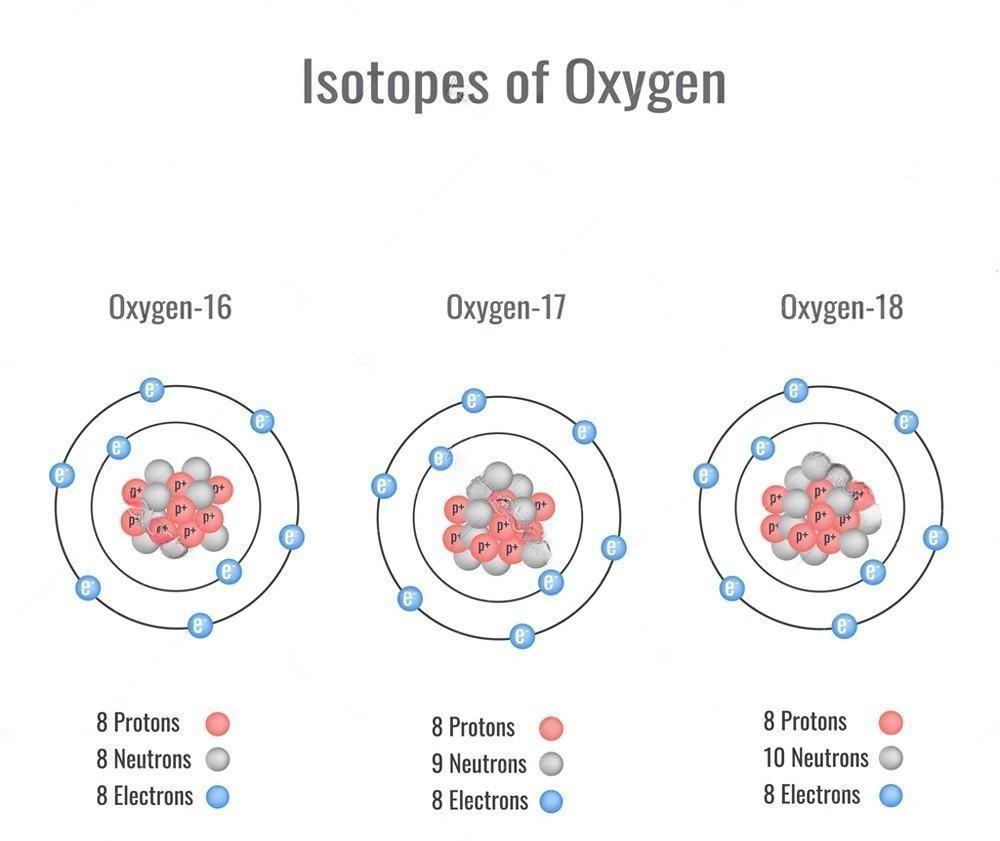
In nature, oxygen exists in the form of 16O, 17O, and 18O with a relative abundance of 99.756%, 0.039%, and 0.205%, respectively. An oxygen isotope is widely used in earth science to determine the source and temperature of diagenetic ore-forming materials. It has a wide application prospects in biology and medicine.
First of all, in drug research, oxygen is an essential element in most drug structures. In metabolic studies, oxygen-18 is usually used to replace common oxygen for isotope tracers, which can sensitively, accurately, and quickly understand the activity rules and metabolic pathways of drugs in the body. Such tracer studies are essential for the development of new drugs and have important implications for guiding drug use in humans.
The second is the application in the study of energy metabolism: the most efficient and accurate method to determine the energy metabolism of free people is to use the double-labeled water technology (DLW), namely the hydrogen and oxygen dual isotope tracer method. Metabolic status can be determined by feeding a human or animal a dose of H-2 (deuterium) and 0-18 double-labeled water and measuring the rate of the isotope excreted from the body. Dual-labeled water technology has been widely used in sports medicine, child nutrition, food nutrition, weight loss, astronaut diet, and other aspects, and has broad prospects for development.
Thirdly, PET (positron emission tomography) technology developed in recent years has provided unprecedented opportunities for the application of heavy oxygen water. Compared with imaging techniques such as CT and nuclear magnetic resonance, which diagnose diseases from the perspective of morphology, PET is known as “in vivo biochemical imaging” because it provides information on the function, metabolism, or receptor binding of lesions at the molecular level. Because the biochemical changes of diseases are often earlier than the morphological changes, it can be a more early, sensitive, and accurate diagnosis of diseases, especially tumors, coronary heart disease, and brain diseases. More than 90% of PET developer is fluorine-18, which is formed by proton bombardment of oxygen-18 in the accelerator. Therefore, as a positron emission target has become the primary use of oxygen-18.
The Isotope of Nitrogen: Nitrogen-15 (15N)
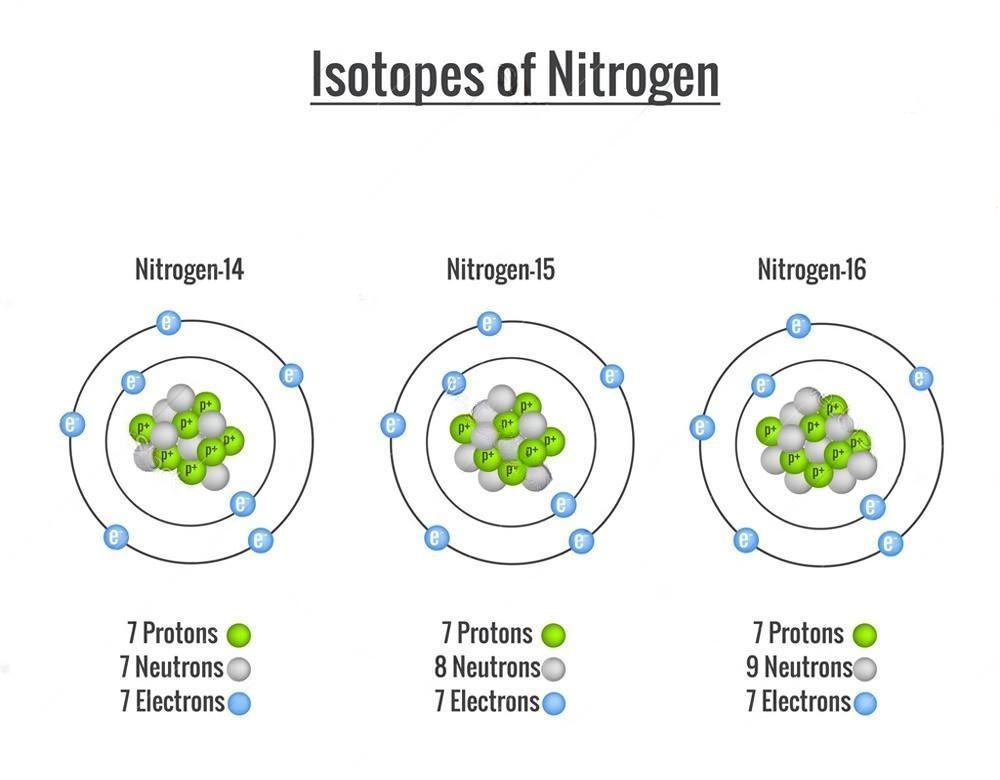
Nitrogen-15 is a rare isotope of nitrogen. The two sources of nitrogen-15 formation are positron emission from oxygen-15 and beta decay from carbon-15. Nitrogen-15 has one of the lowest neutron capture cross sections of all isotopes.
Nitrogen-15 is frequently used in NMR (nitrogen-15 nuclear magnetic resonance spectroscopy). Unlike the more common nitrogen-14, which has integer spin and therefore quadrupole moments, the spin of 15N is not an integer (3/2). This provides NMR with advantages, such as narrower line widths. Nitrogen-15 tracking is one way to study the nitrogen cycle.
Which field is Isotopically Labeled Compounds used for?
The 6th International Symposium on Synthesis and Application of Isoturbulence and Isoturbulence Labeled Compounds was held in Philadelphia, USA from 14 to 18 September 1997. More than 400 people from more than 20 countries and regions attended the conference. More than 200 papers were published on the application and labeling of more than 30 isotropic compounds. About 30% of the compounds are short-lived isotopes C and F. Long-lived isotopes “C, H” and stable isotope D (Deuterium) account for about 50%. Since most compounds, especially organic compounds and biological compounds, contain carbon and hydrogen, the radioactive isotope of these two elements is used to label tracers, to ensure that the labeled compounds have exactly the same structure as the pre-labeled compounds. Therefore, in the synthesis and application of labeled compounds, the isotopes of hydrogen and carbon are the most widely used.
Application of Isotopically Labeled Compounds in the medical field
Stable isotope products have been widely used in clinical research in the medical field, diagnosis and identification of a variety of diseases, condition judgment, therapeutic effect evaluation, organ function research, and new drug development, such as PET diagnosis, 13C-urea expiratory detection, tumor therapy (boron neutron capture therapy), drug research, etc.
Application of Isotopically Labeled Compounds in pharmaceutical scientific research
The application of isotope-labeled compounds in the field of pharmacy mainly focuses on the study of pharmacology, toxicology, and pharmacokinetics. It is widely used in organic and bioorganic synthesis, polymer (protein, nucleic acid, monoclonal antibody), and other fields. The application in pharmacology mainly focuses on the basic research of pharmacological and biochemical mechanisms, such as systemic metabolism, bone calcium kinetics, anti-specific pharmacokinetics, the study of stable isotopes with biochemical drug tracers, and the calculation of continuous flow and isotope ratio of labeled compounds, and the binding of radiopharmaceuticals in hemoglobin, etc.
Application of Isotopically Labeled Compounds in the field of life science
The study of the structure and function of different protein populations by nuclear magnetic resonance (NMR) and mass spectrometry (MS) requires stable isotope integration techniques, including isotope coding affinity labeling (ICAT™), stable isotope labeling of amino acids in cell culture (SILAC), and absolute quantitative analysis of target proteins (AQUA™). Stable isotopes are introduced into proteins and other biological macromolecules through biological metabolism, enzymatic hydrolysis, or chemical introduction. The structure diagram of biological macromolecules can be obtained by using molecular biology software after analysis with large instruments.
Application of Isotopically Labeled Compounds in the field of energy metabolism
Energy metabolism research focuses on sports medicine, childhood nutrition, food nutrition as well as weight loss, and astronaut diet. The stable isotope tracer method is one of the methods to study metabolism, and the commonly used stable isotopes include 2H, 15N, 13C, 18O, etc. For example, the 2H and 18O doubly-labeled water (DLW) technology is a new way to evaluate the human body’s energy consumption. This method has been preliminarily applied in laboratory and site studies in sports science and is the most accurate way to evaluate energy metabolism at present.
Application of Isotopically Labeled Compounds in agricultural scientific research
Stable isotopes 15N and 13C are widely used in plant physiology and biochemistry research, soil and plant nutrition research, plant protection research, rice, flowers, agricultural products and other crop improvement research, grassland nitrogen cycle research, and so on. Most stable isotope products used in agriculture are low-abundance products. Inorganic salts with low abundance such as 15N-labeled urea, 15N-labeled ammonium sulfate, 15N-labeled ammonium nitrate, and 15N-labeled ammonium chloride are commonly used as fertilizer tracers.