What Are the Uses of Enantiomers in Our Lives?
Abstract
Chemical enantiomers, defined as non-superimposable mirror images, play a vital role across various scientific and industrial domains due to their distinct biological and chemical properties. This paper explores the significance of enantiomers in drug development, highlighting their impact on therapeutic efficacy and safety, as illustrated by the cases of thalidomide and ibuprofen. Additionally, the applications of enantiomers in agriculture and the food and flavor industry are examined, emphasizing their importance in improving efficiency and product quality. Recent advancements in separation techniques and the development of new chiral catalysts are discussed, showcasing the progress in producing high-purity enantiomers. Furthermore, innovative applications in materials science and environmental science are considered, expanding the potential uses of enantiomers. The paper concludes with an outlook on future research directions, focusing on enhancing synthesis, separation, and application methods while integrating green chemistry principles to ensure sustainability.
Introduction to enantiomers
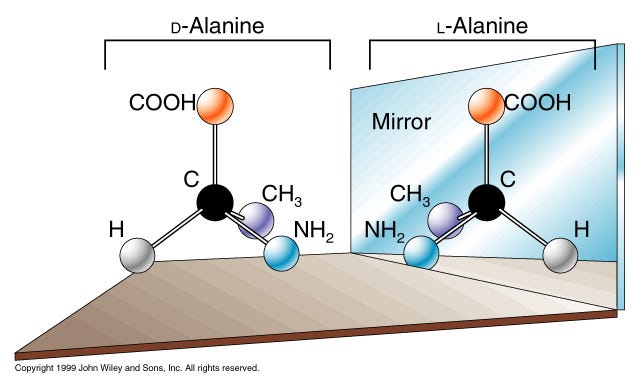
Chemical enantiomers are an intriguing and essential aspect of chemistry and biology, playing a pivotal role in various scientific fields and everyday applications. Enantiomers are a type of stereoisomer, where two molecules are non-superimposable mirror images of each other. This characteristic leads to significant differences in how these molecules interact with biological systems, making the study of enantiomers crucial for fields such as pharmacology, biochemistry, and organic chemistry.
In chemistry, the concept of chirality is fundamental to understanding enantiomers. A molecule is considered chiral if it cannot be superimposed on its mirror image. This lack of symmetry typically arises from the presence of a chiral center, most often a carbon atom bonded to four distinct substituents. The existence of chiral centers in molecules leads to the formation of two enantiomers, each of which is a mirror image of the other but cannot be aligned perfectly with its counterpart.
What are Chemical Enantiomers?
Definition and Explanation
Chemical enantiomers are a fascinating aspect of molecular science, defined as pairs of molecules that are non-superimposable mirror images of each other. These molecules possess the same molecular formula and sequence of bonded atoms but differ in their three-dimensional orientations. The concept of enantiomers is deeply rooted in the notion of chirality, which describes objects that cannot be superimposed on their mirror images. In chemistry, chirality arises when a molecule contains a chiral center, usually a carbon atom bonded to four different substituents.
Chirality and Chiral Centers
A chiral center is the heart of chirality in molecules. When a carbon atom is attached to four distinct groups, it creates two possible arrangements that are mirror images of each other. These arrangements are the enantiomers. The presence of a chiral center ensures that the molecule and its mirror image cannot be superimposed, similar to how a left hand cannot be superimposed on a right hand. This property is crucial in understanding the behavior and interactions of enantiomers in various environments.
Non-Superimposable Mirror Images
The concept of non-superimposability is essential for distinguishing enantiomers. Imagine two gloves, one for the left hand and one for the right hand. Although they are identical in shape and structure, they cannot be perfectly aligned on top of each other. This analogy helps in visualizing the non-superimposable nature of enantiomers. Each enantiomer in a pair will have a unique spatial arrangement that mirrors the other, yet they remain distinct entities.
Importance of Enantiomers in Chemistry and Biology
The study of enantiomers extends beyond mere structural curiosity; it has profound implications for biological systems. One of the most striking examples of this is the different effects enantiomers can have on living organisms. Enantiomers can exhibit dramatically different biological activity due to their distinct three-dimensional shapes. This difference in activity is crucial in pharmacology, where one enantiomer of a drug might have therapeutic effects, while the other could be inactive or even harmful.
A historical example highlighting the importance of enantiomeric purity is the case of thalidomide. In the 1950s and 1960s, thalidomide was prescribed as a sedative and anti-nausea medication for pregnant women. However, it was later discovered that while one enantiomer of thalidomide had the desired effects, the other caused severe birth defects. This tragedy underscored the necessity of understanding and controlling enantiomeric purity in pharmaceuticals.
In addition to pharmaceuticals, enantiomers are significant in other industries such as agriculture, where they are used in the production of pesticides and herbicides. The enantiomeric form of these chemicals can influence their effectiveness and environmental impact. In the food and flavor industry, enantiomers can affect the taste and smell of products, highlighting their importance in ensuring quality and consumer satisfaction.
Chemical enantiomers are a critical area of study in chemistry and biology due to their unique properties and diverse applications. Understanding the nature of enantiomers, their biological significance, and their practical uses can lead to advancements in various scientific and industrial fields, emphasizing the need for continued research and development in this area.
Optical Activity
Explanation of Optical Rotation
One of the most distinctive properties of enantiomers is their optical activity, which refers to their ability to rotate plane-polarized light. When plane-polarized light passes through a solution containing an enantiomer, the plane of polarization is rotated. The direction and degree of this rotation depend on the nature of the enantiomer and its concentration. Optical rotation is a key characteristic used to distinguish between different enantiomers.
Dextrorotatory vs. Levorotatory
Enantiomers are classified based on the direction in which they rotate plane-polarized light. If an enantiomer rotates light in a clockwise direction, it is termed dextrorotatory and is denoted by the symbol (+). Conversely, if it rotates light counterclockwise, it is called levorotatory and is denoted by the symbol (-). The optical activity of enantiomers is an important parameter in various scientific and industrial applications, as it helps identify and differentiate between enantiomers.
Measuring Optical Activity
The measurement of optical activity is conducted using a polarimeter, an instrument that measures the angle of rotation caused by passing polarized light through a chiral substance. This measurement provides valuable information about the enantiomeric composition of a sample and is widely used in quality control and research in pharmaceuticals, food, and other industries where enantiomers play a critical role.
Biological Significance of Enantiomers
Different Effects of Enantiomers in Biological Systems
Enantiomers can have markedly different effects in biological systems due to their unique three-dimensional arrangements. This distinction arises because biological molecules, such as enzymes and receptors, are also chiral and can interact differently with each enantiomer. One enantiomer of a compound may fit well into a receptor site and elicit a strong biological response, while its mirror image may fit poorly or not at all, leading to a significantly different or even opposite effect.
For example, the enantiomers of the drug thalidomide have vastly different biological activities. One enantiomer was effective as a sedative and anti-nausea medication, while the other caused severe birth defects. This tragic case highlighted the critical need for understanding and controlling enantiomeric purity in pharmaceuticals. Similarly, the enantiomers of ibuprofen, a common pain reliever, exhibit different levels of biological activity, with only one enantiomer providing therapeutic effects.
Examples of Enantiomers in Pharmaceuticals
In the pharmaceutical industry, the significance of enantiomers cannot be overstated. Many drugs are administered as racemic mixtures, containing both enantiomers. However, advances in chiral chemistry have allowed for the development of single-enantiomer drugs, which can offer enhanced efficacy and reduced side effects. For instance, the antidepressant drug citalopram is marketed as a racemic mixture, but its enantiomer escitalopram is available as a single-enantiomer drug with greater efficacy and fewer side effects.
Case Studies: Thalidomide, Ibuprofen, etc.
The case of thalidomide is perhaps the most well-known example of the importance of enantiomers. Prescribed in the 1950s and 1960s to alleviate morning sickness in pregnant women, it was later discovered that while one enantiomer had the desired sedative effects, the other caused severe teratogenic effects, leading to birth defects. This case led to stricter regulations and a deeper understanding of chiral drugs in the pharmaceutical industry.
Ibuprofen, another widely used drug, exists as two enantiomers, but only the S-enantiomer is biologically active as an anti-inflammatory agent. The R-enantiomer is inactive but can be converted to the active form in the body. The development and marketing of single-enantiomer versions of drugs like ibuprofen have improved therapeutic outcomes and reduced adverse effects.
Separation and Resolution of Enantiomers
Methods for Separating Enantiomers
Separating enantiomers from a racemic mixture, known as resolution, is a critical process in the production of chiral drugs. Several methods are employed to achieve this, including chiral chromatography, crystallization, and the use of chiral resolving agents. Chiral chromatography involves the use of a chiral stationary phase to separate enantiomers based on their differential interactions with the phase. This method is widely used in both research and industrial settings for its effectiveness and precision.
Chiral Chromatography
Chiral chromatography is one of the most effective techniques for enantiomer separation. By utilizing a chiral stationary phase, this method exploits the different interactions each enantiomer has with the chiral environment, resulting in their separation. This technique is invaluable in the pharmaceutical industry for ensuring the purity and efficacy of chiral drugs.
Chiral Resolving Agents
Another method for separating enantiomers involves the use of chiral resolving agents. These agents form diastereomeric complexes with the enantiomers, which can then be separated due to their different physical properties. This method is particularly useful in large-scale industrial applications where chiral chromatography may not be feasible.
Practical Applications
Enantiomers in Drug Development
Enantiomers play a pivotal role in drug development, profoundly impacting the efficacy and safety of pharmaceutical compounds. The presence of chirality in drug molecules can lead to significant differences in the biological activity of each enantiomer. This is because biological systems themselves are chiral, meaning that the interaction between drug molecules and biological targets (such as enzymes, receptors, and nucleic acids) can vary dramatically between enantiomers.
The development of single-enantiomer drugs has become a crucial focus in pharmaceutical research. Single-enantiomer drugs often provide better therapeutic outcomes and reduce the risk of adverse effects. For example, the enantiomer escitalopram, derived from the racemic mixture of citalopram, is more effective as an antidepressant and has fewer side effects. This targeted approach not only enhances drug efficacy but also improves patient safety and compliance.
Enantiomers in Agriculture
Enantiomers also have significant applications in agriculture, particularly in the development of pesticides and herbicides. The biological activity of these chemicals can vary greatly between enantiomers. Utilizing the more active enantiomer can lead to more efficient pest control with reduced environmental impact. For example, the herbicide metolachlor exists as a mixture of enantiomers, but the S-enantiomer is more effective at controlling weed growth. By using the single, active enantiomer, farmers can achieve better results with lower quantities of the chemical, thereby minimizing environmental contamination and reducing costs.
Enantiomers in the Food and Flavor Industry
In the food and flavor industry, enantiomers are essential for creating specific tastes and aromas. Many natural and artificial flavors are chiral, and the enantiomers can have distinctly different sensory properties. For instance, the R-enantiomer of carvone has a spearmint flavor, while the S-enantiomer has a caraway flavor. Understanding and utilizing the correct enantiomer is crucial for producing the desired taste and aroma in food products. This precision enhances product quality and consumer satisfaction, demonstrating the broad relevance of enantiomers beyond pharmaceuticals.
Recent Advances and Research
Latest Research in Enantiomer Separation
The field of enantiomer separation has seen significant advancements, driven by the need for pure enantiomers in pharmaceuticals and other industries. One of the most promising areas of research is the development of new chiral catalysts and ligands that can facilitate asymmetric synthesis. These catalysts are designed to selectively produce one enantiomer over the other, streamlining the production process and improving yield.
Another exciting development is the use of advanced chromatography techniques. High-performance liquid chromatography (HPLC) with chiral stationary phases has become a standard method for separating enantiomers. Innovations in column technology and the development of new chiral selectors continue to enhance the efficiency and resolution of these separations, making them more accessible and practical for large-scale applications.
Innovative Applications of Enantiomers
Beyond traditional fields, enantiomers are finding innovative applications in new and emerging technologies. In the field of materials science, chiral molecules are being used to create novel materials with unique optical and electronic properties. For example, chiral liquid crystals are used in advanced display technologies, and chiral polymers are being explored for use in enantioselective sensors and catalysts.
In environmental science, chiral pollutants and their effects are being studied to develop better remediation strategies. Understanding how different enantiomers of pollutants interact with biological systems can lead to more effective cleanup methods and reduce the ecological impact of these substances.
Future Directions in Enantiomer Research
The future of enantiomer research holds great promise, with ongoing studies aimed at improving synthesis, separation, and application methods. Advances in computational chemistry and molecular modeling are providing deeper insights into chiral interactions, guiding the design of more efficient catalysts and separation techniques. Additionally, the integration of green chemistry principles in enantiomer production and application is becoming increasingly important, aiming to reduce environmental impact and enhance sustainability.
The continued exploration of enantiomers will likely lead to new breakthroughs in various fields, from medicine and agriculture to materials science and beyond. As our understanding of chirality and its applications grows, the potential for innovation and improvement in numerous industries remains vast and exciting.
Conclusion
The study of chemical enantiomers is a crucial and dynamic field that intersects various scientific disciplines, including chemistry, biology, pharmaceuticals, agriculture, and materials science. Enantiomers, as non-superimposable mirror images, exhibit unique properties that can lead to vastly different biological and chemical activities. This characteristic is particularly significant in drug development, where the correct enantiomer can enhance therapeutic efficacy and reduce adverse effects, as evidenced by the cases of thalidomide and ibuprofen.
In agriculture, the use of single-enantiomer pesticides and herbicides can improve efficiency and minimize environmental impact, while in the food and flavor industry, the precise application of enantiomers ensures the desired sensory qualities. These applications underscore the importance of understanding and utilizing enantiomers in various industrial contexts.
Recent advances in enantiomer separation techniques, such as high-performance liquid chromatography and the development of new chiral catalysts, have significantly improved the ability to produce and isolate pure enantiomers. These technological advancements facilitate the production of high-purity enantiomers essential for pharmaceuticals and other industries.
Innovative applications of enantiomers in materials science and environmental science further expand the potential uses of these fascinating molecules. The development of chiral materials with unique optical and electronic properties, as well as the study of chiral pollutants, represents exciting frontiers in enantiomer research.
Future directions in enantiomer research are likely to focus on enhancing synthesis, separation, and application methods. The integration of green chemistry principles will be pivotal in ensuring sustainable practices in the production and use of enantiomers. As computational chemistry and molecular modeling advance, they will provide deeper insights into chiral interactions, guiding the development of more efficient and sustainable technologies.
Overall, the study and application of chemical enantiomers hold immense promise for innovation and improvement across numerous fields, making continued research and development in this area both essential and exciting.
References
- Clayden, J., Greeves, N., Warren, S., & Wothers, P. (2012). Organic Chemistry. Oxford University Press.
- Smith, M. B. (2016). March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (8th ed.). Wiley.
- Eliel, E. L., & Wilen, S. H. (1994). Stereochemistry of Organic Compounds. Wiley.
- Silverman, R. B., & Holladay, M. W. (2014). The Organic Chemistry of Drug Design and Drug Action (3rd ed.). Elsevier.
- Ward, T. J., & Ward, K. D. (2012). Chiral separations: A review of current topics and trends. Analytica Chimica Acta, 731, 1-18.