Understanding Roxadustat: Uses, Effectiveness, and Risks
Abstract
Roxadustat is a medication prescribed for the treatment of anemia in individuals with chronic kidney disease. Its favorable therapeutic outcomes in clinical trials have resulted in its approval for use in several countries, such as the United States, China, and Japan. As an inhibitor of hypoxic inducer prolyl hydroxylase, it can inhibit HIF-PHD activity and promote the production of erythropoietin, thereby increasing the content of red blood cells and achieving the purpose of treating anemia. Roxadustat may provide a more versatile treatment option for a wider range of anemic patients than erythropoietic agents. Overall, Roxadustat is a promising treatment option for anemia in patients with CKD. Our company provides high-quality Roxadustat and related products for scientific research. We hope that more researchers can find more application fields of Roxadustat and create value for human health.
What is Roxadustat?
Roxadustat (CAS:808118-40-3) is a small molecule inhibitor that promotes erythropoietin production by inhibiting HIF-prolyl hydroxylase. Erythropoietin can promote red blood cell production, and thus achieve the purpose of anemia. Roxadustat is an oral drug that has been approved for use in several countries, including the United States, Japan, and China, only for patients with anemia due to chronic kidney disease.
Treatment of CKD patients with Roxadustat
Mechanism of action:
Roxadustat, a hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitor (PHI), is utilized to treat anemia in patients with chronic kidney disease (CKD). Its mechanism of action involves the stimulation of erythropoietin (EPO) production and the subsequent increase in red blood cell (RBC) production in the body.
In normal conditions, HIF-α is hydroxylated by PHIs, which leads to its degradation via the proteasome pathway. This prevents HIF-α from accumulating in the cell, which is important for maintaining homeostasis. However, in hypoxic conditions, such as in CKD, HIF-α is stabilized and translocates to the nucleus where it binds to hypoxia response elements (HREs) and activates the transcription of target genes, including EPO.
Roxadustat inhibits PHIs, which prevents the degradation of HIF-α, even in normoxic conditions. This leads to the accumulation of HIF-α in the cell, which stimulates the transcription of target genes, including EPO. The increase in EPO leads to an increase in RBC production, which helps to improve anemia in CKD patients.
In addition to stimulating EPO production, Roxadustat also promotes the differentiation of erythroid progenitor cells, which further increases the production of RBCs. This effect is believed to be mediated by the direct activation of HIF-α in erythroid progenitor cells.
Overall, the mechanism of action of Roxadustat involves the inhibition of PHIs, leading to the stabilization and accumulation of HIF-α in the cell. This activates the transcription of target genes, including EPO, and promotes the differentiation of erythroid progenitor cells, which ultimately leads to an increase in RBC production and an improvement in anemia in patients with CKD[1].
Clinical trials:
Clinical trials have been conducted to evaluate its safety and efficacy in improving anemia in this patient population.
Two clinical trials have evaluated the efficacy and safety of Roxadustat in treating anemia in patients with chronic kidney disease (CKD). The first trial was a phase 2[2], randomized, double-blind, placebo-controlled study that enrolled 154 non-dialysis-dependent CKD patients with anemia. The trial found that Roxadustat was effective in increasing hemoglobin levels compared to placebo and was generally well tolerated. The second trial was a phase 3, randomized, open-label, active-controlled study that enrolled 1,043 CKD patients undergoing dialysis[3]. The study compared the efficacy of Roxadustat to epoetin alfa, a commonly used erythropoiesis-stimulating agent, and found that Roxadustat was non-inferior in increasing hemoglobin levels and was generally well tolerated.
Roxadustat has shown positive results in clinical trials for anemia in patients with CKD and has been approved for marketing in several countries. However, further studies are needed to confirm its long-term safety, efficacy, and efficacy compared to existing treatments.
Adverse effects:
While Roxadutat has been shown to be effective in clinical trials, it can also cause adverse effects.
The most commonly reported adverse effects of Roxadustat including hypertension, diarrhea, nausea, vomiting, and nasopharyngitis. These side effects were found to be generally mild to moderate in severity and did not result in treatment discontinuation in most cases.
In rare cases, however, Roxadustat can cause more serious adverse effects such as cardiovascular events, thromboembolic events, and hypersensitivity reactions. These events were more commonly reported in patients with a history of cardiovascular disease or thrombotic events. In clinical trials, Roxadustat was also associated with a higher incidence of malignancy, although it is unclear whether the drug is directly responsible for this increased risk.
Patients receiving Roxadustat should be monitored for adverse effects, particularly those with a history of cardiovascular disease or thrombotic events. Close monitoring of blood pressure is also recommended, as Roxadustat can increase blood pressure in some patients[4].
To sum up, although Roxadustat may effectively treat anemia in patients with chronic kidney disease, careful monitoring is crucial to detect any possible adverse effects, especially in those with prior cardiovascular or thrombotic complications.
Comparison to other anemia treatments:
Roxadustat is a novel treatment for anemia in chronic kidney disease (CKD) patients that works by activating the hypoxia-inducible factor (HIF) pathway, leading to increased erythropoietin production and red blood cell synthesis. Compared to other anemia treatments, such as erythropoiesis-stimulating agents (ESAs) and iron supplements, Roxadustat offers several potential advantages.
Firstly, Roxadustat can be administered orally, while ESAs and iron supplements are typically administered by injection or intravenous infusion. This could make Roxadustat more convenient for patients who prefer oral medication or have difficulty accessing healthcare facilities for regular injections[5].
Additionally, Roxadustat has demonstrated its efficacy in treating anemia in both dialysis and non-dialysis patients with CKD, whereas erythropoiesis-stimulating agents (ESAs) are only authorized for use in CKD patients receiving dialysis. This may render Roxadustat a more adaptable therapeutic alternative for a broader spectrum of anemic patients.
Thirdly, Roxadustat has been shown to increase iron mobilization and reduce the need for intravenous iron supplementation in CKD patients, while ESAs typically require additional iron supplementation to be effective. This could potentially reduce the risk of iron overload and associated complications in CKD patients.
However, Roxadustat also has some potential disadvantages compared to ESAs. For example, Roxadustat has been associated with a higher incidence of adverse events such as hypertension, cardiovascular events, and malignancy in some clinical trials. In addition, Roxadustat is a newer treatment option and its long-term safety and efficacy profile is not yet fully understood[6].
In conclusion, Roxadustat offers some potential advantages over traditional anemia treatments such as ESAs and iron supplements, including oral administration, effectiveness in both dialysis and non-dialysis CKD patients, and reduced need for intravenous iron supplementation. However, the potential risks and uncertainties associated with roxadustat should also be carefully considered when choosing an appropriate treatment for anemia in CKD patients.
Potential use of Roxadustat in other medical conditions
Ongoing research is exploring the potential use of Roxadustat in other medical conditions, although it is mainly used for the treatment of anemia in patients with chronic kidney disease (CKD).
For example, there is research suggesting that Roxadustat may be effective in treating anemia associated with myelodysplastic syndromes (MDS)[7], a group of disorders in which the bone marrow does not produce enough healthy blood cells. Roxadustat is also being investigated for the treatment of anemia associated with cancer, particularly in patients undergoing chemotherapy.
In addition to its potential use for treating anemia, there is research exploring the potential therapeutic applications of Roxadustat in other medical conditions, such as heart failure and pulmonary hypertension[8]. However, it should be noted that these potential uses are still in the experimental stages and further research is needed to determine their safety and effectiveness.
Overall, while roxadustat is primarily used for treating anemia in CKD patients, ongoing research is exploring its potential uses in other medical conditions as well.
Patent protection of Roxadustat
Roxadustat is a patented drug, and the patent protection for Roxadustat is held by FibroGen, a biopharmaceutical company that developed the drug[9]. In the United States, FibroGen has multiple patents covering the composition of matter, methods of use, and methods of synthesis of roxadustat. The earliest of these patents was filed in 2008 and is set to expire in 2028, although other patents covering roxadustat have later expiration dates.
In other countries, the patent protection for roxadustat may vary depending on local laws and regulations. For example, in China, the patent protection for roxadustat is held by AstraZeneca, which holds the commercial rights to the drug in China through a partnership with FibroGen. The Chinese patent covering the use of roxadustat for treating anemia in patients with CKD was issued in 2015 and is set to expire in 2032.
It is worth noting that patent protection can have a significant impact on the availability and cost of medications, as it can limit competition from generic versions of the drug. However, once the patent protection for roxadustat expires, it may become more widely available and affordable to patients.
Synthesis of Roxadustat
Due to its proven efficacy as an active compound, Roxadustat has been successfully marketed. Moreover, ongoing research into its synthesis method has resulted in the discovery of additional synthesis routes, expanding the options available for its production (Scheme 1)[10-16].
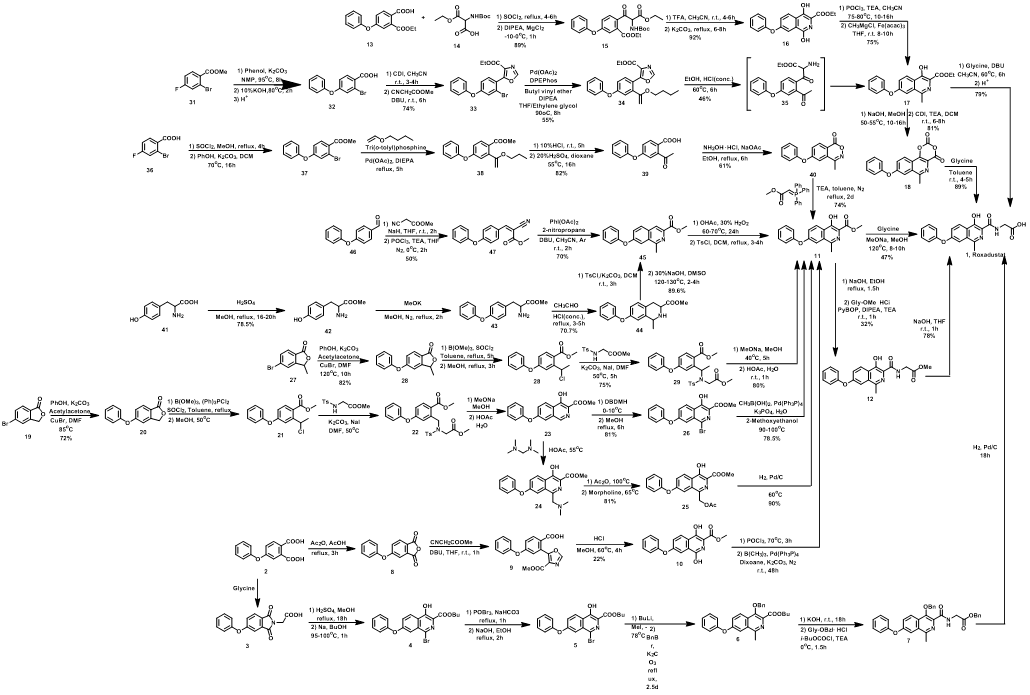
Scheme 1 Synthesis Route of Roxadustat
The synthesis of Roxadustat usually starts with 4-phenoxybenzaldehyde, and reacts with methyl cyanoacetate in alkaline conditions to produce methyl (Z) -2-Cyano-3 -(4-phenoxyphenyl)acrylate, Further, the DBU catalyzed free radical reaction, intramolecular cyclization, quinoline derivatives. Further reactions introduce hydroxyl and glycine fragments to produce Roxadustat.
The synthesis of Roxadustat is a complex and challenging process that requires careful control of reaction conditions and precise manipulation of chemical intermediates. It is typically carried out on a large scale in industrial settings by trained chemists and chemical engineers.
Overall, the chemical synthesis of Roxadustat is a testament to the ingenuity and creativity of synthetic chemists, who are able to design and carry out complex chemical reactions to create new and life-saving medications.
Reference:
Provenzano, R., Besarab, A., Sun, C. H., Diamond, S. A., Durham, J. H., Cangiano, J. L., … & Neff, T. B. (2016). Oral hypoxia–inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clinical Journal of the American Society of Nephrology, 11(6), 982-991.
Akizawa, T., Iwasaki, M., Otsuka, T., Reusch, M., & Misumi, T. (2019). Roxadustat treatment of chronic kidney disease-associated anemia in Japanese patients not on dialysis: a phase 2, randomized, double-blind, placebo-controlled trial. Advances in Therapy, 36, 1438-1454.
Akizawa, T., Otsuka, T., Reusch, M., & Ueno, M. (2020). Intermittent oral dosing of roxadustat in peritoneal dialysis chronic kidney disease patients with anemia: a randomized, phase 3, multicenter, open‐label study. Therapeutic Apheresis and Dialysis, 24(2), 115-125.
Qie, S., Jiao, N., Duan, K., Li, J., Liu, Y., & Liu, G. (2021). The efficacy and safety of roxadustat treatment for anemia in patients with kidney disease: a meta-analysis and systematic review. International urology and nephrology, 53, 985-997.
Chen, N., Hao, C., Liu, B. C., Lin, H., Wang, C., Xing, C., … & Yu, K. H. P. (2019). Roxadustat treatment for anemia in patients undergoing long-term dialysis. New England Journal of Medicine, 381(11), 1011-1022.
Fu, Z., Geng, X., Chi, K., Song, C., Wu, D., Liu, C., & Hong, Q. (2022). Efficacy and safety of daprodustat vs rhEPO for anemia in patients with chronic kidney disease: a meta-analysis and trial sequential analysis. Frontiers in pharmacology, 13.
Henry, D. H., Glaspy, J., Harrup, R. A., Mittelman, M., Zhou, A., Carraway, H. E., … & Yu, P. (2020). Oral roxadustat demonstrates efficacy in anemia secondary to lower-risk myelodysplastic syndrome irrespective of ring sideroblasts and baseline erythropoietin levels. Blood, 136, 29-30.
Weir, M. R. (2021). Managing anemia across the stages of kidney disease in those hyporesponsive to erythropoiesis-stimulating agents. American Journal of Nephrology, 52(6), 450-466.
FibroGen, Inc. (2019). Patent landscape report on roxadustat. World Intellectual Property Organization.
Arend, M., Flippin, L., Guenzler-Pukall, V., Ho, W. B., Turtle, E., & Du, X. (2007). U.S. Patent Application No. 11/549,571.
Hill, J. H. (1965). Mechanism of the Gabriel—Colman Rearrangement. The Journal of Organic Chemistry, 30(2), 620-622.
Kang, X., Long, W., Zhang, J., Hu, Y., & Wang, Y. (2015). U.S. Patent No. 9,206,134. Washington, DC: U.S. Patent and Trademark Office.
SUZUKI, M., NUNAMI, K. I., MATSUMOTO, K., YONEDA, N., & MIYOSHI, M. (1978). A Facile Synthesis of 1-Oxo-1, 2-dihydroisoquinoline-3-carboxylate and 2-Pyridone-6-carboxylate Derivatives. Synthesis, 1978(06), 461-462.
Witschi, C., Park, J. M., Thompson, M. D., Martinelli, M. J., Yeowell, D. A., & Arend, M. P. (2014). U.S. Patent No. 8,883,823. Washington, DC: U.S. Patent and Trademark Office.
Píša, O., Rádl, S., Čerňa, I., & Šembera, F. (2021). A Scalable Synthesis of Roxadustat (FG-4592). Organic Process Research & Development, 26(3), 915-924.
Oae, S., Kitao, T., & Kitaoka, Y. (1963). The mechanisms of the reactions of p-toluenesulfonyl chloride with isoquinoline-and pyridine n-oxides. Tetrahedron, 19(6), 827-832.
Lu, S. C., Li, H. S., Gong, Y. L., Zhang, S. P., Zhang, J. G., & Xu, S. (2018). Combination of PhI (OAc) 2 and 2-nitropropane as the source of methyl radical in room-temperature metal-free oxidative decarboxylation/cyclization: construction of 6-methyl phenanthridines and 1-methyl isoquinolines. The Journal of Organic Chemistry, 83(24), 15415-15425.