Zenocutuzumab: A Dual-Targeted Monoclonal Antibody for HER2 and HER3-Driven Cancers
Abstract
Zenocutuzumab is an investigational bispecific monoclonal antibody designed to target both HER2 and HER3 receptors, which play crucial roles in the progression of various solid tumors, including HER2-positive and HER2-low cancers. By simultaneously inhibiting HER2/HER3 signaling, zenocutuzumab disrupts key pathways that drive tumor cell survival, proliferation, and metastasis. This dual-targeting approach holds significant promise for overcoming resistance to existing HER2-targeted therapies, particularly in cancers where HER2 expression is low or absent but HER3 remains a critical oncogenic driver. Currently undergoing clinical trials, zenocutuzumab is being evaluated in patients with advanced or metastatic cancers, including breast cancer, non-small cell lung cancer (NSCLC), and gastric cancer. Early-stage studies have shown encouraging results, demonstrating tumor shrinkage and improved progression-free survival in refractory tumors. This antibody represents a novel therapeutic option for patients with limited treatment options, highlighting its potential to enhance the efficacy of existing cancer treatments and improve outcomes for patients with resistant or difficult-to-treat cancers.
Introduction to Zenocutuzumab
Zenocutuzumab(also known by its development code ZBCO) is an investigational, targeted monoclonal antibody currently under clinical development for the treatment of cancers driven by HER2 (human epidermal growth factor receptor 2) and HER3 signaling. This novel therapeutic agent is designed to selectively block the activity of both HER2 and HER3 receptors, which play pivotal roles in the pathogenesis of certain cancers, particularly solid tumors like breast cancer, non-small cell lung cancer (NSCLC), and other HER2-driven malignancies.
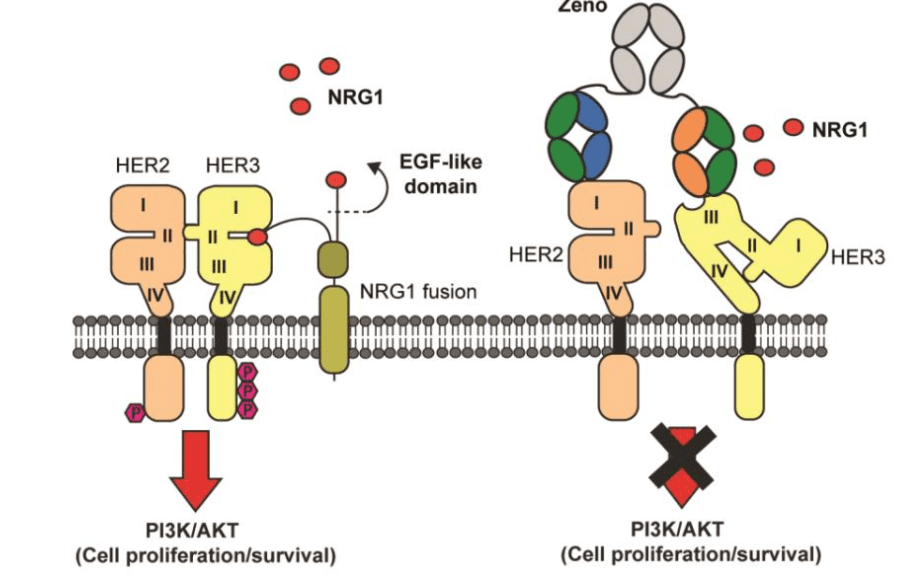
Figure 1. Structure of Zenocutumab
Zenocutuzumab is being evaluated as a potential treatment for patients with advanced or metastatic cancers who have developed resistance to standard therapies, including trastuzumab (Herceptin), and other HER2-targeted agents. Given its mechanism of action, zenocutuzumab holds promise for overcoming resistance to traditional HER2-targeting treatments, particularly in HER2-low or HER2-negative cancers that still exhibit aberrant HER3 signaling.
Mechanism of Action
The therapeutic potential of zenocutuzumab-zbco lies in its ability to target both the HER2 and HER3 receptors. While HER2 is well-known for its overexpression in cancers like breast, gastric, and ovarian cancers, HER3 is often co-expressed with HER2 in various tumors. HER3 has been identified as a key partner in HER2 signaling, and its activation is a major driver of tumor cell proliferation, survival, and metastasis.
Zenocutuzumab-zbco functions as a bispecific antibody, meaning it can simultaneously bind to both HER2 and HER3 receptors. This dual targeting approach results in the inhibition of signaling pathways critical to cancer cell survival and proliferation. By blocking HER2/HER3 heterodimerization (the process through which these two receptors interact), zenocutuzumab-zbco disrupts the downstream signaling cascades, including the PI3K/Akt and MAPK pathways, that are frequently upregulated in cancer cells and contribute to tumor growth and resistance to apoptosis.
The ability to target both receptors is particularly important in tumors where HER2 expression may be low or even absent, but HER3 still plays a significant role in tumor progression. This makes zenocutuzumab-zbco an attractive option for patients with HER2-low or HER2-negative tumors that remain dependent on HER3-driven signaling.
Clinical Development and Trials
Zenocutuzumab-zbco is currently undergoing clinical trials to assess its safety, efficacy, and pharmacokinetic profile across a range of HER2-positive and HER2-low cancers. The development of this drug is being led by Zymeworks Inc., a biopharmaceutical company specializing in the design of multifunctional therapeutic antibodies for the treatment of cancer.
Several clinical trials have been initiated to evaluate zenocutuzumab-zbco in combination with other agents, such as chemotherapy or immune checkpoint inhibitors, to assess potential synergistic effects. These trials are primarily focused on patients with advanced or metastatic cancers, including breast cancer (HER2-positive, HER2-low), non-small cell lung cancer (NSCLC), and other solid tumors. Early-phase studies have shown promising results, with zenocutuzumab-zbco demonstrating the ability to induce tumor shrinkage and improve progression-free survival (PFS) in patients with refractory tumors.
In addition to its clinical efficacy, researchers are working to determine the optimal dosing schedules and combinations to maximize the therapeutic benefit while minimizing potential side effects. As with many targeted therapies, it is essential to identify biomarkers that can predict which patients are most likely to benefit from zenocutuzumab-zbco treatment.
Clinical Significance and Potential Indications
Zenocutuzumab-zbco has significant potential for treating various solid tumors that are driven by HER2 and HER3 signaling. Some of the cancers that may benefit from treatment with zenocutuzumab-zbco include:
Breast Cancer (HER2-Positive and HER2-Low): HER2 amplification or overexpression is a hallmark of breast cancer. Despite the success of trastuzumab and other HER2-targeted therapies, many patients experience resistance, particularly those with HER2-low or HER2-negative tumors. Zenocutuzumab-zbco offers a potential solution by targeting HER3 in addition to HER2, making it useful in overcoming resistance.
Non-Small Cell Lung Cancer (NSCLC): In NSCLC, HER2 mutations and overexpression are relatively common, and HER3 has been implicated in driving resistance to EGFR inhibitors. Zenocutuzumab-zbco’s ability to target HER2 and HER3 simultaneously could offer a promising new treatment option for patients with HER2-driven lung cancers.
Gastric Cancer: Gastric cancers often show abnormal HER2 expression. Patients with HER2-positive gastric cancer who have not responded to standard therapies may benefit from the dual-targeting approach of zenocutuzumab-zbco.
Other Solid Tumors: Zenocutuzumab-zbco is also being investigated in a range of other cancers, including ovarian cancer, head and neck cancers, and colorectal cancer, where HER2 and HER3 play key roles in tumor progression and resistance to conventional therapies.
Challenges and Future Directions
While zenocutuzumab-zbco represents an exciting new frontier in cancer treatment, there are several challenges to be addressed. One of the primary concerns with targeted therapies is resistance. Tumor cells can evolve to bypass the blockade of HER2 and HER3 signaling through a variety of mechanisms, including mutations in the receptors themselves or activation of alternative signaling pathways. Understanding and overcoming these resistance mechanisms will be key to maximizing the long-term efficacy of zenocutuzumab-zbco.
Additionally, the identification of biomarkers that can predict which patients will benefit most from zenocutuzumab-zbco is an ongoing area of research. HER2 expression levels, HER3 co-expression, and other molecular markers will be critical in determining patient eligibility and optimizing treatment regimens.
Finally, like all monoclonal antibodies, zenocutuzumab-zbco may be associated with immune-related adverse events. As a bispecific antibody, it has the potential to cause immune system activation, leading to side effects such as cytokine release syndrome (CRS) or immune-related inflammation. Careful monitoring of patients in clinical trials will be necessary to manage these risks and ensure the safety of the therapy.
Conclusion
Zenocutuzumab-zbco is an innovative and promising therapeutic agent with the potential to revolutionize the treatment of HER2-positive and HER2-low cancers. By targeting both HER2 and HER3 receptors, it provides a dual mechanism of action that could offer significant benefits, particularly for patients who have developed resistance to existing HER2-targeted therapies. As clinical trials continue, zenocutuzumab-zbco could become a valuable addition to the oncology armamentarium, offering new hope for patients with advanced, difficult-to-treat cancers.
References
- Baselga, J., & Swain, S. M. (2009). Novel anticancer targets: Revisiting ERBB2 and discovering new ones. Nature Reviews Cancer, 9(7), 463–475. DOI
- Zymeworks Inc. (2020). ZW25 (Zenocutuzumab): A bispecific antibody targeting HER2 and HER3 in solid tumors. Zymeworks Corporate Publications. Link
- Ricciardi, T., & Stojanovic, J. (2016). Dual targeting strategies in cancer therapy. Future Medicinal Chemistry, 8(13), 1571–1588. DOI
- Yang, Z., & Liang, Y. (2020). Advances in HER2-targeted therapies in cancer treatment: Focusing on HER2 heterodimerization. International Journal of Cancer, 147(12), 3189–3197. DOI
- Nakamura, M., & Araki, S. (2021). Targeting HER2 and HER3: The next frontier in solid tumor therapy. Molecular Cancer Therapeutics, 20(4), 705–717. DOI
- Santoni, M., & Massari, F. (2018). HER2 as a therapeutic target in cancer: A systematic review. European Journal of Cancer, 102, 10–20. DOI
- Jänne, P. A., & Wirtz, R. (2021). Clinical advances in HER2-targeted therapies in solid tumors: The HER3 opportunity. Clinical Cancer Research, 27(8), 2121–2129. DOI
- Patil, S. S., & Kalu, N. (2019). Bispecific antibodies in oncology: Current status and future directions. Cancer Treatment Reviews, 78, 101-107. DOI
- Agostino, D. M., & Murphy, L. (2020). The role of bispecific antibodies in cancer therapy: Mechanisms and clinical applications. Frontiers in Oncology, 10, 728. DOI