Zevtera: A Broad-Spectrum Solution for Treating Resistant Bacterial Infections
Abstract
Zevtera (ceftobiprole medocaril) is a fifth-generation cephalosporin antibiotic with broad-spectrum efficacy against Gram-positive and Gram-negative bacteria, including methicillin-resistant Staphylococcus aureus (MRSA). It is primarily used for treating complicated skin and soft tissue infections (cSSTI) and hospital-acquired pneumonia (HAP), excluding ventilator-associated pneumonia (VAP). Zevtera’s mechanism of action involves inhibiting bacterial cell wall synthesis by binding to penicillin-binding proteins (PBPs), even in resistant bacteria. It has shown great promise in addressing the growing threat of antibiotic resistance. While generally well tolerated, Zevtera can cause gastrointestinal disturbances, allergic reactions, and Clostridioides difficile-associated diarrhea (CDAD). Appropriate patient monitoring and adherence to antibiotic stewardship principles are essential to maximizing its therapeutic potential. This review highlights Zevtera’s clinical applications, safety profile, and role in combating resistant bacterial infections.
Introduction to Zevtera
Zevtera (ceftobiprole medocaril) is a fifth-generation cephalosporin antibiotic developed to address some of the most challenging bacterial infections in clinical settings. Approved for the treatment of complicated skin and soft tissue infections (cSSTI) and hospital-acquired pneumonia (HAP), excluding ventilator-associated pneumonia (VAP), Zevtera stands out due to its broad-spectrum efficacy against both Gram-positive and Gram-negative bacteria. This versatility makes it a valuable option, especially when dealing with multidrug-resistant pathogens.
Fig.1 Zevtera’s Structure
One of Zevtera’s most significant advantages is its activity against methicillin-resistant Staphylococcus aureus (MRSA), a notorious cause of hospital-acquired infections. In addition, its effectiveness against Gram-negative organisms, including Escherichia coli and Pseudomonas aeruginosa, further broadens its clinical application. Zevtera’s dual action against both Gram-positive and Gram-negative bacteria gives it a critical role in situations where other antibiotics may fail, particularly in settings where bacterial resistance is prevalent.
Zevtera works by inhibiting bacterial cell wall synthesis, which leads to bacterial cell death. This mechanism, while common to cephalosporins, is enhanced by ceftobiprole’s strong binding affinity to key penicillin-binding proteins (PBPs) involved in bacterial cell wall formation. This feature allows Zevtera to be effective even in bacteria with advanced resistance mechanisms.
As the global threat of antibiotic resistance continues to rise, medications like Zevtera offer new hope in the fight against hard-to-treat infections. Its use in treating infections caused by resistant bacteria is vital for improving patient outcomes and reducing the spread of resistant strains. Zevtera’s broad-spectrum activity and effectiveness against resistant pathogens make it a cornerstone of modern antibacterial therapy.
Mechanism of Action
Zevtera (ceftobiprole medocaril) exerts its antibacterial effects by targeting the bacterial cell wall, a critical structure for bacterial survival. Like other β-lactam antibiotics, Zevtera binds to and inhibits penicillin-binding proteins (PBPs), which play an essential role in the synthesis of peptidoglycan, the primary component of the bacterial cell wall. Without a functioning cell wall, bacteria become structurally compromised, leading to cell lysis and death. This action makes Zevtera a bactericidal agent, capable of directly killing bacteria rather than merely inhibiting their growth.
What sets Zevtera apart from earlier generations of cephalosporins is its strong affinity for a broader range of PBPs, including those found in resistant bacteria like methicillin-resistant Staphylococcus aureus (MRSA) and some Gram-negative organisms. Zevtera’s high binding affinity to PBP2a, a protein that confers methicillin resistance in MRSA, is a key feature of its mechanism. This allows the drug to overcome one of the primary resistance mechanisms in MRSA infections. Furthermore, Zevtera’s activity extends to Gram-negative bacteria, including Escherichia coli and Pseudomonas aeruginosa, which are often associated with hospital-acquired infections.
Another important aspect of Zevtera’s mechanism is its stability against certain β-lactamases, enzymes produced by some bacteria that degrade β-lactam antibiotics. While many antibiotics are rendered ineffective by these enzymes, Zevtera’s structure enables it to maintain activity, making it a more reliable choice for treating infections caused by resistant strains.
Zevtera’s ability to target multiple PBPs and remain effective in the presence of resistance mechanisms highlights its significance in treating multidrug-resistant bacterial infections. Its broad-spectrum efficacy and targeted mechanism of action make it a valuable tool in managing both Gram-positive and Gram-negative bacterial infections.
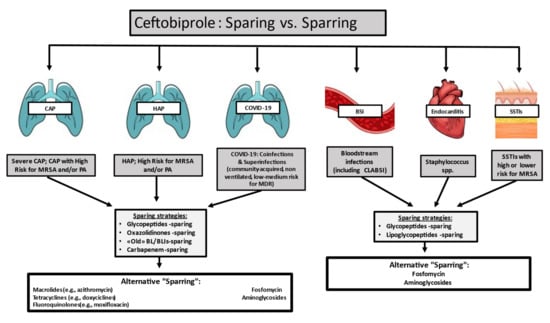
Fig.2 Ceftobiprole stewardship perspective
Therapeutic Applications of Zevtera
Zevtera (ceftobiprole medocaril) is primarily indicated for the treatment of complicated skin and soft tissue infections (cSSTI) and hospital-acquired pneumonia (HAP), excluding ventilator-associated pneumonia (VAP). Its broad-spectrum efficacy against both Gram-positive and Gram-negative bacteria makes it an important option in settings where multidrug-resistant organisms are common, particularly in hospitals and healthcare facilities.
Complicated Skin and Soft Tissue Infections (cSSTI)
Zevtera is especially effective in the treatment of cSSTI, a category of infections that can include deep tissue involvement, surgical site infections, and infections in patients with underlying comorbidities such as diabetes. One of its key advantages is its activity against methicillin-resistant Staphylococcus aureus (MRSA), which is a frequent cause of these infections. MRSA can be difficult to treat with traditional antibiotics due to its resistance to many β-lactam antibiotics, but Zevtera’s ability to target PBP2a, the protein responsible for MRSA’s methicillin resistance, makes it particularly valuable.
Additionally, Zevtera’s efficacy extends to other Gram-positive bacteria, such as Streptococcus pyogenes and Streptococcus agalactiae, which are common causes of skin and soft tissue infections. On the Gram-negative side, its activity against Escherichia coli and Pseudomonas aeruginosa broadens its therapeutic potential, covering a range of infections typically seen in healthcare settings. This makes Zevtera a versatile choice for cSSTI, particularly in patients at high risk of infection with resistant organisms.
Hospital-Acquired Pneumonia (HAP)
Hospital-acquired pneumonia is another critical area where Zevtera has proven to be effective. HAP is a major concern in healthcare settings, especially in intensive care units, where patients are often exposed to a range of bacterial pathogens, many of which are resistant to multiple antibiotics. Zevtera’s ability to treat both Gram-positive bacteria like MRSA and Gram-negative pathogens such as Klebsiella pneumoniae and Pseudomonas aeruginosa makes it a valuable tool in managing these complex infections.
One of the distinguishing features of Zevtera in treating HAP is its broad-spectrum activity, which covers a wide array of bacteria commonly responsible for pneumonia in hospitalized patients. The exclusion of ventilator-associated pneumonia (VAP) from its indications is notable, but in cases of non-ventilator-associated HAP, Zevtera offers a potent option, particularly where bacterial resistance is a concern. Clinical trials have shown that Zevtera has comparable efficacy to other first-line treatments like linezolid and vancomycin but with the added benefit of Gram-negative coverage.
Zevtera and Antibiotic Resistance
Antibiotic resistance is one of the most significant public health challenges of the 21st century, and Zevtera plays an essential role in addressing this issue. The rise of multidrug-resistant organisms, particularly MRSA and resistant Gram-negative bacteria, has made the development of new antibiotics critical for effective patient care. Zevtera’s mechanism of action, which targets penicillin-binding proteins even in resistant bacteria, positions it as a key solution in the fight against antibiotic-resistant infections.
Activity Against MRSA
MRSA infections, particularly in hospitals, pose a major threat due to their resistance to most β-lactam antibiotics. Zevtera’s ability to inhibit PBP2a, a protein responsible for methicillin resistance, gives it a significant advantage over other antibiotics. This makes it an essential option for treating skin infections and pneumonia caused by MRSA. Its use in treating cSSTI and HAP provides a reliable alternative to other MRSA-targeting antibiotics like vancomycin and linezolid, with the added benefit of covering Gram-negative bacteria as well.
Efficacy Against Gram-Negative Bacteria
In addition to its activity against MRSA, Zevtera is also effective against key Gram-negative pathogens, including Pseudomonas aeruginosa and Escherichia coli, both of which are common culprits in hospital-acquired infections. The ability to cover both Gram-positive and Gram-negative bacteria in a single treatment option helps streamline therapy, reducing the need for combination antibiotics and minimizing the risk of developing additional resistance.
Zevtera’s stability against β-lactamase-producing bacteria further enhances its utility in environments where resistance is common. While many antibiotics are inactivated by β-lactamases, enzymes that break down the antibiotic before it can act, Zevtera remains effective, making it a reliable option for treating resistant infections.
Safety and Side Effects of Zevtera
Zevtera (ceftobiprole medocaril) is generally well tolerated, but like all antibiotics, it is associated with certain side effects. Common adverse reactions include gastrointestinal disturbances such as nausea, vomiting, and diarrhea. Patients may also experience headache, rash, or elevated liver enzyme levels, which suggest mild liver irritation. These side effects are typically manageable and often resolve after completing the treatment.
One major concern with Zevtera, as with other β-lactam antibiotics, is the risk of allergic reactions, which can range from mild rashes to severe, life-threatening anaphylaxis. Patients with a known allergy to cephalosporins or penicillins should be carefully monitored during treatment, and in severe cases, alternative antibiotics should be considered.
Another potential complication is Clostridioides difficile-associated diarrhea (CDAD), a serious gastrointestinal condition caused by disruption of the gut microbiota. CDAD can occur during or after antibiotic treatment and may result in severe diarrhea, colitis, or even life-threatening complications like toxic megacolon. If CDAD is suspected, Zevtera should be discontinued, and appropriate therapy for C. difficile should be initiated.
Zevtera is excreted primarily via the kidneys, so dosage adjustments may be necessary in patients with renal impairment to prevent drug accumulation and toxicity. Regular monitoring of renal and liver function is recommended during prolonged treatment to minimize the risk of adverse effects.
Overall, Zevtera’s safety profile is comparable to other β-lactam antibiotics, but close monitoring is essential, particularly in patients with pre-existing conditions or a history of drug allergies.
Conclusion
Zevtera (ceftobiprole medocaril) is a critical antibiotic in the treatment of complicated bacterial infections, particularly in hospital settings where multidrug-resistant organisms pose significant challenges. Its broad-spectrum activity against both Gram-positive bacteria, including MRSA, and Gram-negative pathogens like Pseudomonas aeruginosa makes it highly versatile for treating serious infections such as complicated skin and soft tissue infections (cSSTI) and hospital-acquired pneumonia (HAP).
Zevtera’s mechanism of action, which targets penicillin-binding proteins even in resistant bacteria, enhances its effectiveness in combating these difficult infections. Despite its overall favorable safety profile, careful monitoring is essential to manage potential side effects, including gastrointestinal disturbances, allergic reactions, and Clostridioides difficile-associated diarrhea (CDAD).
As antibiotic resistance continues to rise globally, Zevtera represents a valuable therapeutic option that addresses the growing need for effective treatment of resistant infections. By incorporating Zevtera into targeted therapeutic protocols and adhering to principles of antibiotic stewardship, healthcare providers can improve patient outcomes while minimizing the risk of further resistance development.
References
- Méndez R, Latorre A, González-Jiménez P. Ceftobiprole medocaril. Rev Esp Quimioter. 2022 Apr;35 Suppl 1(Suppl 1):25-27. doi: 10.37201/req/s01.05.2022. Epub 2022 Apr 22. PMID: 35488820; PMCID: PMC9106187.
- Hsu WH, Hsu CK, Lai CC. Ceftobiprole medocaril for the treatment of pneumonia. Expert Rev Anti Infect Ther. 2023 Jun;21(6):551-563. doi: 10.1080/14787210.2023.2202851. Epub 2023 Apr 18. PMID: 37042813.
- Azanza Perea JR, Sádaba Díaz de Rada B. Ceftobiprole: pharmacokinetics and PK/PD profile. Rev Esp Quimioter. 2019 Sep;32 Suppl 3(Suppl 3):11-16. PMID: 31364336; PMCID: PMC6755345.
- Falcó V, Burgos J, Almirante B. Ceftobiprole medocaril for the treatment of community-acquired pneumonia. Expert Opin Pharmacother. 2018 Sep;19(13):1503-1509. doi: 10.1080/14656566.2018.1516749. Epub 2018 Sep 10. PMID: 30198789.
- Xu E, Pérez-Torres D, Fragkou PC, Zahar JR, Koulenti D. Nosocomial Pneumonia in the Era of Multidrug-Resistance: Updates in Diagnosis and Management. Microorganisms. 2021 Mar 5;9(3):534. doi: 10.3390/microorganisms9030534. PMID: 33807623; PMCID: PMC8001201.
- Bassetti M, Vena A, Croxatto A, Righi E, Guery B. How to manage Pseudomonas aeruginosa infections. Drugs Context. 2018 May 29;7:212527. doi: 10.7573/dic.212527. PMID: 29872449; PMCID: PMC5978525.




