Oxygen
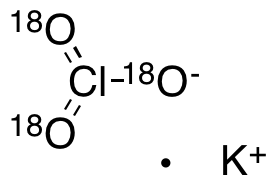
17O and 18O labeling compounds involve replacing the common oxygen isotope, 16O, with either 17O or 18O, accomplished by introducing labeled water or other labeled molecules into a reaction or metabolic pathway. These isotopes have versatile applications. For example, 18O labeling is useful in studying protein and peptide synthesis, allowing researchers to monitor the incorporation of labeled oxygen atoms into the protein's amino acid backbone. Additionally, 18O labeling can be employed in metabolic studies to trace the fate of oxygen atoms in biochemical reactions. Although less common, 17O labeling can be used to study the exchange of oxygen atoms in reactions, such as the exchange of water molecules during photosynthesis in plants.
Furthermore, 17O and 18O labeling are valuable in environmental science, particularly in tracing the source and movement of water in hydrological systems. Overall, 17O and 18O labeling compounds are crucial tools in various research fields, including biochemistry, environmental science, and geology. By replacing oxygen atoms with isotopically labeled versions, researchers can follow the fate of oxygen atoms in reactions and metabolic pathways, providing valuable insights into a range of phenomena.