Home
>
Reference Standards> 1-(4-Aminophenyl)-3-methylcarbamyl-4-methyl-3,4-dihydro-7,8-methylenedioxy-5H-2,3-benzodiazepinehydr
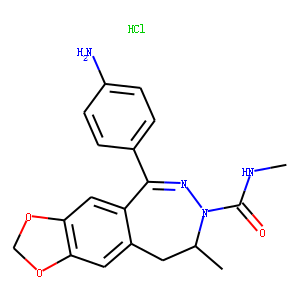 1-(4-Aminophenyl)-3-methylcarbamyl-4-methyl-3,4-dihydro-7,8-methylenedioxy-5H-2,3-benzodiazepinehydr
1-(4-Aminophenyl)-3-methylcarbamyl-4-methyl-3,4-dihydro-7,8-methylenedioxy-5H-2,3-benzodiazepinehydr
For research use only. Not for therapeutic Use.
GYKI53655 Hydrochloride is a non-competitive AMPA and kainate receptor antagonist. GYKI53655 Hydrochloride exhibits anticonvulsant activity. GYKI53655 Hydrochloride also blocks GluK3 homomeric receptors (IC50 = 63 μM) and GluK2b(R)/GluK3 heteroreceptors (IC50 = 32 μM) at high concentrations.
| CAS Number | 143692-48-2 |
| Synonyms | (S)-5-(4-aminophenyl)-N,8-dimethyl-8,9-dihydro-7H-[1,3]dioxolo[4/’,5/’:4,5]benzo[1,2-d][1,2]diazepine-7-carboxamide hydrochloride |
| Molecular Formula | C19H20N4O3.HCl |
| Purity | ≥95% |
| Target | Neuronal Signaling |
| Solubility | Soluble in DMSO > 10 mM |
| Storage | Desiccate at RT |
| InChI | InChI=1S/C19H20N4O3.ClH/c1-11-7-13-8-16-17(26-10-25-16)9-15(13)18(22-23(11)19(24)21-2)12-3-5-14(20)6-4-12;/h3-6,8-9,11H,7,10,20H2,1-2H3,(H,21,24);1H |
| InChIKey | ASLCSBBDVWPSQT-UHFFFAOYSA-N |
| SMILES | CC1N(C(NC)=O)N=C(C2=CC=C(N)C=C2)C3=CC4=C(OCO4)C=C3C1.[H]Cl |
| Reference | </br>1: Miyazaki S, Minami T, Mizuma H, Kanazawa M, Doi H, Matsumura S, Lu J, Onoe H, Furuta K, Suzuki M, Ito S. The action site of the synthetic kainoid (2S,3R,4R)-3-carboxymethyl-4-(4-methylphenylthio)pyrrolidine-2-carboxylic acid (PSPA-4), an analogue of Japanese mushroom poison acromelic acid, for allodynia (tactile pain). Eur J Pharmacol. 2013 Jun 15;710(1-3):120-7. doi: 10.1016/j.ejphar.2012.10.023. PubMed PMID: 23124023.</br>2: Alt A, Weiss B, Ornstein PL, Gleason SD, Bleakman D, Stratford RE Jr, Witkin JM. Anxiolytic-like effects through a GLUK5 kainate receptor mechanism. Neuropharmacology. 2007 Jun;52(7):1482-7. PubMed PMID: 17418283.</br>3: Rodríguez-Moreno A, Sihra TS. Presynaptic kainate receptor-mediated facilitation of glutamate release involves Ca2+-calmodulin and PKA in cerebrocortical synaptosomes. FEBS Lett. 2013 Mar 18;587(6):788-92. doi: 10.1016/j.febslet.2013.01.071. PubMed PMID: 23416300.</br></br> |
| Chemistry Calculators | Dilution Calculator In vivo Formulation Calculator Molarity Calculator Molecular Weight Calculator Reconstitution Calculator |