Home
>
Reference Standards> (+)-9-OXO-11ALPHA,15R-DIHYDROXY-16-(3-(TRIFLUOROMETHYL)PHENOXY)-17,18,19,20-TETRANOR-PROSTA-5Z,13E-D
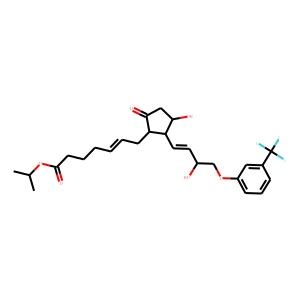 (+)-9-OXO-11ALPHA,15R-DIHYDROXY-16-(3-(TRIFLUOROMETHYL)PHENOXY)-17,18,19,20-TETRANOR-PROSTA-5Z,13E-D
(+)-9-OXO-11ALPHA,15R-DIHYDROXY-16-(3-(TRIFLUOROMETHYL)PHENOXY)-17,18,19,20-TETRANOR-PROSTA-5Z,13E-D
For research use only. Not for therapeutic Use.
Fluprostenol is a well-<wbr></wbr>studied, potent analog of prostaglandin F<sub>2α</sub> (PGF<sub>2α</sub>) and acts primarily through the FP receptor. Oxidation at C-<wbr></wbr>9 of fluprostenol yields 9-<wbr></wbr>keto fluprostenol. Prostaglandin esters are known to be hydrolyzed in the eye to the corresponding free acids. However, the use of prostaglandin esters as prodrugs outside the eye is relatively unexplored. 9-<wbr></wbr>keto Fluprostenol is an analog of PGE<sub>2</sub> with structural modifications intended to give it a prolonged half-<wbr></wbr>life and greater potency. 9-<wbr></wbr>keto Fluprostenol isopropyl ester has the potential to act as an EP agonist in prodrug form. However, no studies on the pharmacology of this compound have been published to date. In addition 9-<wbr></wbr>keto fluprostenol isopropyl ester is a potential metabolite of Travoprost, which is the Alcon trade name for fluprostenol isopropyl ester. In monkey cornea, this transformation was observed as a product of NADP Certain F-<wbr></wbr>series prostaglandins have been shown to be converted to the corresponding E-<wbr></wbr>series compounds in rabbit liver and human platelet preparations.
| CAS Number | 1219032-18-4 |
| Synonyms | Fluprostenol Prostaglandin E2 |
| Molecular Formula | C26H33F3O6 |
| Purity | ≥95% |
| Storage | -20°C |
| IUPAC Name | propan-2-yl (Z)-7-[(1R,2R,3R)-3-hydroxy-2-[(E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]but-1-enyl]-5-oxocyclopentyl]hept-5-enoate |
| InChI | InChI=1S/C26H33F3O6/c1-17(2)35-25(33)11-6-4-3-5-10-21-22(24(32)15-23(21)31)13-12-19(30)16-34-20-9-7-8-18(14-20)26(27,28)29/h3,5,7-9,12-14,17,19,21-22,24,30,32H,4,6,10-11,15-16H2,1-2H3/b5-3-,13-12+/t19-,21-,22-,24-/m1/s1 |
| InChIKey | OKXNVRDMSRTJEN-FFKVAXMCSA-N |
| SMILES | CC(C)OC(=O)CCCC=CCC1C(C(CC1=O)O)C=CC(COC2=CC=CC(=C2)C(F)(F)F)O |
| Chemistry Calculators | Dilution Calculator In vivo Formulation Calculator Molarity Calculator Molecular Weight Calculator Reconstitution Calculator |