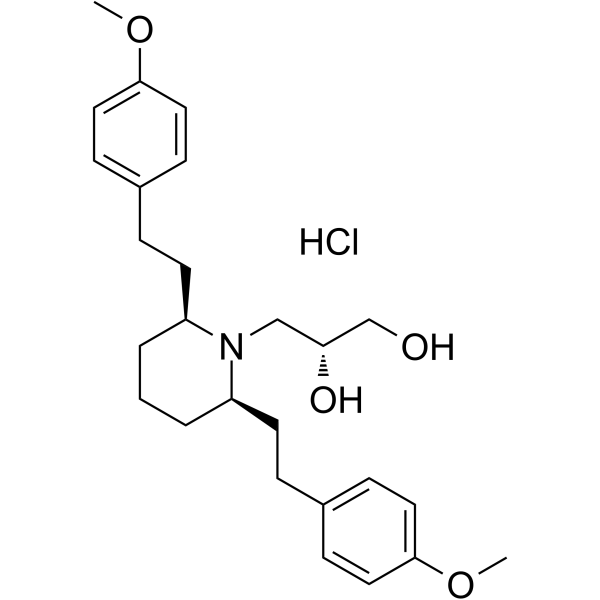
GZ-793A(Cat No.:I012586)is an investigational compound being developed as a potent and selective inhibitor of the enzyme receptor-interacting protein kinase 1 (RIPK1). RIPK1 plays a critical role in regulating cell death pathways, including necroptosis and inflammation. By targeting RIPK1, GZ-793A aims to block excessive cell death and inflammation associated with various diseases, such as neurodegenerative disorders, autoimmune diseases, and ischemic injury. Its ability to modulate these pathways makes it a promising candidate for therapeutic intervention, offering potential benefits in conditions where uncontrolled cell death contributes to disease progression.