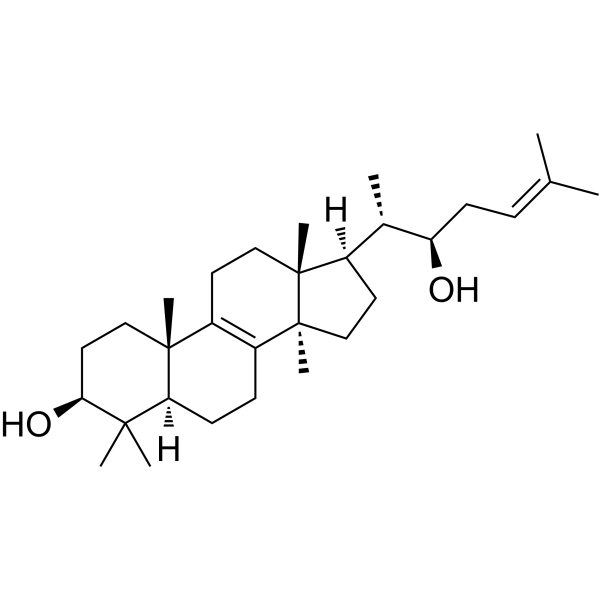
Inotodiol(Cat No.:I029381)is a bioactive triterpenoid compound found in the medicinal mushroom Inonotus obliquus (chaga). Known for its potent antioxidant, anti-inflammatory, and anticancer properties, Inotodiol has been studied for its potential therapeutic benefits. It has shown promise in modulating immune responses, reducing oxidative stress, and inhibiting the growth of various cancer cell types. Additionally, Inotodiol’s ability to support overall health by protecting cells from damage and promoting cellular repair makes it a valuable compound in natural medicine. Its applications are being explored in cancer therapies, immune modulation, and general wellness.