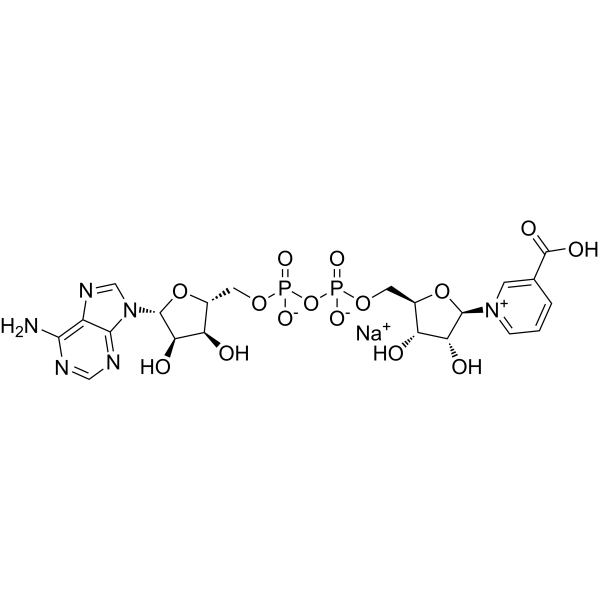
NAAD sodium (Cat No.:M008195) is a naturally occurring analog of NAD⁺, involved in redox reactions and cellular metabolism. It plays a role in nicotinic acid metabolism and serves as a coenzyme in various enzymatic reactions related to energy production, DNA repair, and signaling pathways. NAAD is also studied for its involvement in calcium signaling and as an intermediate in the biosynthesis of NAD⁺. The sodium salt form improves solubility and stability, making it suitable for biochemical assays and research focused on metabolism, aging, and NAD⁺-related therapeutic interventions.